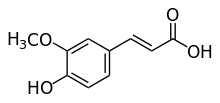
Ferulic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(E)-3-(4-hydroxy-3-methoxy-phenyl)prop-2-enoic acid | |
| Other names
2-propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)- ferulic acid 3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid 3-(4-hydroxy-3-methoxyphenyl)acrylic acid 3-methoxy-4-hydroxycinnamic acid 4-hydroxy-3-methoxycinnamic acid (2E)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid Ferulate Coniferic acid trans-ferulic acid (E)-ferulic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.013.173 |
PubChem CID |
|
| |
| |
| Properties | |
| C10H10O4 | |
| Molar mass | 194.18 g/mol |
| Appearance | Crystalline Powder |
| Melting point | 168 to 172 °C (334 to 342 °F; 441 to 445 K) |
| 0.78 g/L[1] | |
| Acidity (pKa) | 4.61[1] |
| Hazards | |
| NFPA 704 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ferulic acid is a hydroxycinnamic acid, an organic compound. It is an abundant phenolic phytochemical found in plant cell walls, covalently bonded as side chains to molecules such as arabinoxylans. As a component of lignin, ferulic acid is a precursor in the manufacture of other aromatic compounds. The name is derived from the genus Ferula, referring to the giant fennel (Ferula communis).
Occurrence in nature
As a building block of lignocelluloses, such as pectin[2] and lignin, ferulic acid is ubiquitous in the plant kingdom.
In food
Ferulic acid is found in a number of vegetable sources, and occurs in particularly high concentrations in popcorn and bamboo shoots.[3][4] It is a major metabolite of chlorogenic acids in humans along with caffeic and isoferulic acid, and is absorbed in the small intestine, whereas other metabolites such as dihydroferulic acid, feruloylglycine and dihydroferulic acid sulfate are produced from chlorogenic acid in the large intestine by the action of gut flora.[5]
In cereals, ferulic acid is localized in the bran – the hard outer layer of grain. In wheat, phenolic compounds are mainly found in the form of insoluble bound ferulic acid and may be relevant to resistance to wheat fungal diseases.[6] The highest known concentration of ferulic acid glucoside has been found in flaxseed (4.1 ± 0.2 g/kg).[7] It is also found in barley grain.[8]
Asterid eudicot plants can also produce ferulic acid. The tea brewed from the leaves of yacón (Smallanthus sonchifolius), a plant traditionally grown in the northern and central Andes, contains quantities of ferulic acid. In legumes, the white bean variety navy bean is the richest source of ferulic acid among the common bean (Phaseolus vulgaris) varieties.[9] It is also found in horse grams (Macrotyloma uniflorum).[10]
Although there are many sources of ferulic acid in nature, its bioavailability depends on the form in which it is present: free ferulic acid has limited solubility in water, and hence poor bioavailability. In wheat grain, ferulic acid is found bound to cell wall polysaccharides, allowing it to be released and absorbed in the small intestine.[11]
In herbal medicines
Ferulic acid has been identified in Chinese medicine herbs such as Angelica sinensis (female ginseng), Cimicifuga heracleifolia[12] and Ligusticum chuangxiong. It is also found in the tea brewed from the European centaury (Centaurium erythraea), a plant used as a medical herb in many parts of Europe.[13]
In processed foods
Cooked sweetcorn releases increased levels of ferulic acid.[14] As plant sterol esters, this compound is naturally found in rice bran oil, a popular cooking oil in several Asian countries.[15]
Ferulic acid glucoside can be found in commercial breads containing flaxseed.[16] Rye bread contains ferulic acid dehydrodimers.[17]
Metabolism

Biosynthesis
Ferulic acid is biosynthesized in plants from caffeic acid by the action of the enzyme caffeate O-methyltransferase.[18]
Ferulic acid, together with dihydroferulic acid, is a component of lignocellulose, serving to crosslink the lignin and polysaccharides, thereby conferring rigidity to the cell walls.[19]
It is an intermediate in the synthesis of monolignols, i.e., the monomers of lignin, and is also used for the synthesis of lignans.
Biodegradation
Ferulic acid is converted by certain strains of yeast, notably strains used in brewing of wheat beers, such as Saccharomyces delbrueckii (Torulaspora delbrueckii), to 4-vinyl guaiacol (2-methoxy-4-vinylphenol) which gives beers such as Weissbier and Wit their distinctive "clove" flavour. Saccharomyces cerevisiae (dry baker's yeast) and Pseudomonas fluorescens are also able to convert trans-ferulic acid into 2-methoxy-4-vinylphenol.[20] In P. fluorescens, a ferulic acid decarboxylase has been isolated.[21]
Ecology
Ferulic acid is one of the compounds that initiate the vir (virulence) region of Agrobacterium tumefaciens, inducing it to infect plant cells.[22]
Extraction
It can be extracted from wheat bran and maize bran using concentrated alkali.[23]
Bio-medical considerations
Ferulic acid, like many natural phenols, is an antioxidant in vitro in the sense that it is reactive toward free radicals such as reactive oxygen species (ROS). ROS and free radicals are implicated in DNA damage, cancer, and accelerated cell aging.
If added to a topical preparation of ascorbic acid and vitamin E, ferulic acid may reduce oxidative stress and formation of thymine dimers in skin.[24] There is also a small amount of research showing oral supplements of ferulic acid can inhibit melanin production in the process of skin whitening.[25]
Other applications
Mass spectrometry
It is used as a matrix for proteins in MALDI mass spectrometry analyses.[26]
See also
- Caffeic acid
- Coumaric acid
- Diferulic acids
- Eugenol
- Isoferulic acid, an isomer of ferulic acid
References
- 1 2 Mota, Fátima L.; Queimada, António J.; Pinho, Simão P.; Macedo, Eugénia A. (August 2008). "Aqueous Solubility of Some Natural Phenolic Compounds". Industrial & Engineering Chemistry Research. 47 (15): 5182–5189. doi:10.1021/ie071452o.
- ↑ Saulnier, Luc; Thibault, Jean-François (1 March 1999). "Ferulic acid and diferulic acids as components of sugar-beet pectins and maize bran heteroxylans". Journal of the Science of Food and Agriculture. 79 (3): 396–402. doi:10.1002/(SICI)1097-0010(19990301)79:3<396::AID-JSFA262>3.0.CO;2-B.
- ↑ Zhao, Zhaohui; Moghadasian, Mohammed H. (August 2008). "Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review". Food Chemistry. 109 (4): 691–702. doi:10.1016/j.foodchem.2008.02.039.
- ↑ Kumar, Naresh; Pruthi, Vikas (December 2014). "Potential applications of ferulic acid from natural sources". Biotechnology Reports. 4: 86–93. doi:10.1016/j.btre.2014.09.002.
- ↑ Bagchi, Debasis; Moriyama, Hiroyoshi; Swaroop, Anand (2016). Green Coffee Bean Extract in Human Health. CRC Press. p. 92. ISBN 9781315353982. Retrieved 23 September 2017.
- ↑ Gelinas, Pierre; McKinnon, Carole M. (2006). "Effect of wheat variety, farming site, and bread-baking on total phenolics". International Journal of Food Science and Technology. 41 (3): 329–332. doi:10.1111/j.1365-2621.2005.01057.x.
- ↑ Beejmohun, Vickram; Fliniaux, Ophélie (2007). "Microwave-assisted extraction of the main phenolic compounds in flaxseed". Phytochemical Analysis. 18 (4): 275–285. doi:10.1002/pca.973.
- ↑ Quinde-Axtell, Zory; Baik, Byung-Kee (2006). "Phenolic Compounds of Barley Grain and Their Implication in Food Product Discoloration". J. Agric. Food Chem. 54 (26): 9978–9984. doi:10.1021/jf060974w.
- ↑ Luthria, Devanand L.; Pastor-Corrales, Marcial A. (2006). "Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties". Journal of Food Composition and Analysis. 19: 205–211. doi:10.1016/j.jfca.2005.09.003.
- ↑ Kawsar, S. M. A.; Huq, E.; Nahar, N.; Ozeki, Y. (2008). "Identification and Quantification of Phenolic Acids in Macrotyloma uniflorum by Reversed Phase-HPLC". American Journal of Plant Physiology. 3 (4): 165–172. doi:10.3923/ajpp.2008.165.172.
- ↑ Anson, Nuria Mateo; van den Berg, Robin; Bast, Aalt; Haenen, Guido R. M. M. (2009). "Bioavailability of ferulic acid is determined by its bioaccessibility". Journal of Cereal Science. 49 (2): 296–300. doi:10.1016/j.jcs.2008.12.001.
- ↑ Sakai, S.; Kawamata, H.; Kogure, T.; Mantani, N.; Terasawa, K.; Umatake, M.; Ochiai, H. (1999). "Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264.7 cells". Mediators of Inflammation. 8 (3): 173–175. doi:10.1080/09629359990513. PMC 1781798. PMID 10704056.
- ↑ Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P. B.; Seabra, R. M.; Bastos, M. L. (2001). "Antioxidant Activity ofCentaurium erythraeaInfusion Evidenced by Its Superoxide Radical Scavenging and Xanthine Oxidase Inhibitory Activity". Journal of Agricultural and Food Chemistry. 49 (7): 3476–3479. doi:10.1021/jf001145s. PMID 11453794.
- ↑ "Cooking sweet corn boosts its ability to fight cancer and heart disease by freeing healthful compounds, Cornell scientists find". Cornell News. Retrieved 2009-09-07.
- ↑ Orthoefer, F. T. (2005). "Chapter 10: Rice Bran Oil". In Shahidi, F. Bailey's Industrial Oil and Fat Products. 2 (6th ed.). John Wiley & Sons, Inc. p. 465. ISBN 978-0-471-38552-3. Retrieved 2012-03-01.
- ↑ Strandås, C.; Kamal-Eldin, A.; Andersson, R.; Åman, P. (2008). "Phenolic glucosides in bread containing flaxseed". Food Chemistry. 110 (4): 997–999. doi:10.1016/j.foodchem.2008.02.088.
- ↑ Boskov Hansen, H.; Andreasen, M.; Nielsen, M.; Larsen, L.; Knudsen, Bach K.; Meyer, A.; Christensen, L.; Hansen, Å. (2014). "Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making". European Food Research and Technology. 214 (1): 33–42. doi:10.1007/s00217-001-0417-6. ISSN 1438-2377.
- ↑ Shahadi, Fereidoon; Naczk, Marian (2004). Phenolics in Food and Nutraceuticals. Florida: CRC Press. p. 4. ISBN 1-58716-138-9.
- ↑ Iiyama, K.; Lam, T. B.-T.; Stone, B. A. (1994). "Covalent Cross-Links in the Cell Wall". Plant Physiology. 104 (2): 315–320. doi:10.1104/pp.104.2.315. ISSN 0032-0889. PMC 159201.
- ↑ Huang, Z.; Dostal, L.; Rosazza, J. P. (1993). "Microbial transformations of ferulic acid by Saccharomyces cerevisiae and Pseudomonas fluorescens". Applied and Environmental Microbiology. 59 (7): 2244–2250. PMC 182264. PMID 8395165.
- ↑ Huang, Z.; Dostal, L.; Rosazza, J. P. (1994). "Purification and characterization of a ferulic acid decarboxylase from Pseudomonas fluorescens". Journal of Bacteriology. 176 (19): 5912–5918. doi:10.1128/jb.176.19.5912-5918.1994. PMC 196807. PMID 7928951.
- ↑ Kalogeraki, Virginia S.; Zhu, Jun; Eberhard, Anatol; Madsen, Eugene L.; Winans, Stephen C. (November 1999). "The phenolic vir gene inducer ferulic acid is O-demethylated by the VirH2 protein of an Agrobacterium tumefaciens Ti plasmid". Molecular Microbiology. 34 (3): 512–522. doi:10.1046/j.1365-2958.1999.01617.x.
- ↑ Buranov, Anvar U.; Mazza, G. (2009). "Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents". Food Chemistry. 115 (4): 1542–1548. doi:10.1016/j.foodchem.2009.01.059.
- ↑ Lin, F. H.; Lin, J. Y.; Gupta, R. D.; Tournas, J. A.; Burch, J. A.; Selim, M. A.; Monteiro-Riviere, N. A.; Grichnik, J. M.; Zielinski, J.; Pinnell, S. R. (2005). "Ferulic Acid Stabilizes a Solution of Vitamins C and E and Doubles its Photoprotection of Skin". Journal of Investigative Dermatology. 125 (4): 826–832. doi:10.1111/j.0022-202X.2005.23768.x. PMID 16185284.
- ↑ Experimental Dermatology, August 2005, pages 601-608; Bioscience, Biotechnology, and Biochemistry, December 2005, pages 2368-2373; International Journal of Dermatology, August 2004, pages 604-607; Journal of Drugs in Dermatology, July–August 2004, pages 377-381; Facial and Plastic Surgery, February 2004, pages 3-9; Dermatologic Surgery, March 2004, pages 385-388; Journal of Bioscience and Bioengineering, March 2005, pages 272-276; Journal of Biological Chemistry, November 7, 2003, pages 44320-44325; Journal of Agriculture and Food Chemistry, February 2003, pages 1201-1207; International Journal of Cosmetic Science, August 2000, pages 291-303; and Anti-Cancer Research, September–October 1999, pages 3769-3774.
- ↑ Beavis, R. C.; Chait, B. T.; Fales, H. M. (1989). "Cinnamic acid derivatives as matrices for ultraviolet laser desorption mass spectrometry of proteins". Rapid Communications in Mass Spectrometry. 3 (12): 432–435. doi:10.1002/rcm.1290031207. PMID 2520223.
