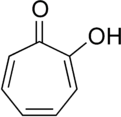
Tropolone
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Hydroxy-2,4,6-cycloheptatrien-1-one | |||
| Other names
2-Hydroxytropone; Purpurocatechol | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.799 | ||
| EC Number | 208-577-2 | ||
| KEGG | |||
| MeSH | D014334 | ||
PubChem CID |
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H6O2 | |||
| Molar mass | 122.12 g/mol | ||
| Melting point | 50 to 52 °C (122 to 126 °F; 323 to 325 K) | ||
| Boiling point | 80 to 84 °C (176 to 183 °F; 353 to 357 K) (0.1 mmHg) | ||
| Acidity (pKa) | 6.89 (and -0.5 for conjugate acid) | ||
| -61·10−6 cm3/mol | |||
| Hazards | |||
| S-phrases (outdated) | S22 S24/25 | ||
| Flash point | 112 °C (234 °F; 385 K) | ||
| Related compounds | |||
Related compounds |
Hinokitiol (4-isopropyl-tropolone) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tropolone is an organic compound with the formula C7H5(OH)O. It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor. Although not usually prepared from tropone, it can be viewed as its derivative with a hydroxyl group in the 2-position.
Synthesis and reactions
Many methods have been described for the synthesis of tropolone.[2] One involves bromination of 1,2-cycloheptanedione with N-bromosuccinimide followed by dehydrohalogenation at elevated temperatures and by acyloin condensation of the ethyl ester of pimelic acid the acyloin again followed by oxidation by bromine.
The compound readily undergoes O-alkylation to give cycloheptatrienyl derivatives, which in turn are versatile synthetic intermediates.[3] With metal cations, it undergoes deprotonation to give chelate complexes, e.g., Cu(O2C7H5)2.
Natural occurrence
Many natural products contain the tropolone skeleton. Among the simplest, and the first to be made synthetically, were α-, β- and γ-thujaplicins, by Ralph Raphael and colleagues.[4] Others include puberulic acid, stipitatonic acid, stipitatic acid, puberulonic acid, sepedonin, and theaflavins of black tea. It arises via a polyketide pathway, which affords a phenolic intermediate that undergoes ring expansion.[3]
Biological effects
It is an inhibitor of grape polyphenol oxidase[5][6] and mushroom tyrosinase.[7]
References
- ↑ Tropolone at Sigma-Aldrich
- ↑ Richard A. Minns "Tropolone" Org. Synth. 1977, volume 57, 117.doi:10.15227/orgsyn.057.0117
- 1 2 Pietra, F. (1973). "Seven-membered conjugated carbo- and heterocyclic compounds and their homoconjugated analogs and metal complexes. Synthesis, biosynthesis, structure, and reactivity". Chemical Reviews. 73: 293–364. doi:10.1021/cr60284a002.
- ↑ Cook, J.W.; Scott, A.I.; Raphael, R.A. (1951). "Tropolones. Part II. The synthesis of α-, β-, and γ-thujaplicins". J. Chem. Soc.: 695–698. doi:10.1039/JR9510000695.
- ↑ Time-dependent inhibition of grape polyphenol oxidase by tropolone. Edelmira Valero, Manuela Garcia-Moreno, Ramon Varon and Francisco Garcia-Carmona, J. Agric. Food Chem., 1991, volume 39, pp. 1043–1046, doi:10.1021/jf00006a007
- ↑ Chedgy, Russell. Secondary metabolites of Western red cedar (Thuja plicata): their biotechnological applications and role in conferring natural durability. LAP Lambert Academic Publishing, 2010, ISBN 3-8383-4661-0, ISBN 978-3-8383-4661-8
- ↑ Inhibition of mushroom tyrosinase by tropolone. Varda Kahn and Andrawis Andrawis, Phytochemistry, Volume 24, Issue 5, 1985, Pages 905-908, doi:10.1016/S0031-9422(00)83150-7