Molybdenum trioxide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Molybdenum trioxide | |||
| Other names | |||
| Identifiers | |||
| ECHA InfoCard | 100.013.823 | ||
PubChem CID |
|||
| Properties | |||
| MoO3 | |||
| Molar mass | 143.95 g·mol−1 | ||
| Appearance | yellow or light blue solid | ||
| Odor | odorless | ||
| Density | 4.69 g/cm3, solid | ||
| Melting point | 795 °C (1,463 °F; 1,068 K) | ||
| Boiling point | 1,155 °C (2,111 °F; 1,428 K) sublimes | ||
| 0.1066 g/100 mL (18 °C) 0.490 g/100 mL (28 °C) 2.055 g/100 mL (70 °C) | |||
| +3.0·10−6 cm3/mol | |||
| Structure | |||
| orthorhombic | |||
| see text | |||
| Thermochemistry | |||
Std molar entropy (S |
77.78 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH |
−745.17 kJ/mol | ||
| Hazards | |||
| Safety data sheet | See: data page | ||
EU classification (DSD) (outdated) |
Carc. Cat. 3 Harmful (Xn) Irritant (Xi) | ||
| R-phrases (outdated) | R36/37, R40 | ||
| S-phrases (outdated) | (S2), S22, S36/37 | ||
| NFPA 704 | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
125 mg.kg (rat, oral) 2689 mg/kg (rat, oral)[1] | ||
LDLo (lowest published) |
120 mg Mo/kg (rat, oral) 120 mg Mo/kg (guinea pig, oral)[1] | ||
LC50 (median concentration) |
>5840 mg/m3 (rat, 4 hr)[1] | ||
| Related compounds | |||
Other cations |
Chromium trioxide Tungsten trioxide | ||
Related molybdenum oxides |
Molybdenum dioxide "Molybdenum blue" | ||
Related compounds |
Molybdic acid Sodium molybdate | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Molybdenum trioxide is chemical compound with the formula MoO3. This compound is produced on the largest scale of any molybdenum compound. It occurs as the rare mineral molybdite. Its chief application is as an oxidation catalyst and as a raw material for the production of molybdenum metal.[2]
Structure
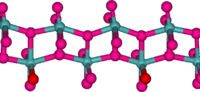
In the gas phase, three oxygen atoms are double bonded to the central molybdenum atom. In the solid state, anhydrous MoO3 is composed of layers of distorted MoO6 octahedra in an orthorhombic crystal. The octahedra share edges and form chains which are cross-linked by oxygen atoms to form layers. The octahedra have one short molybdenum-oxygen bond to a non-bridging oxygen.[3][4]
Preparation and principal reactions
MoO3 is produced industrially by roasting molybdenum disulfide, the chief ore of molybdenum:[2]
- 2 MoS2 + 7 O2 → 2 MoO3 + 4 SO2
The laboratory synthesis of the dihydrate entails acidification of aqueous solutions of sodium molybdate with perchloric acid:[5]
- Na2MoO4 + H2O + 2 HClO4 → MoO3(H2O)2 + 2 NaClO4
The dihydrate loses water readily to give the monohydrate. Both are bright yellow in color.
Molybdenum trioxide dissolves slightly in water to give "molybdic acid". In base, it dissolves to afford the molybdate anion.
Uses
Molybdenum trioxide is used to manufacture molybdenum metal, which serves as an additive to steel and corrosion-resistant alloys. The relevant conversion entails treatment of MoO3 with hydrogen at elevated temperatures:
- MoO3 + 3 H2 → Mo + 3 H2O
It is also a component of the co-catalyst used in the industrial production of acrylonitrile by the oxidation of propene and ammonia.
Because of its layered structure and the ease of the Mo(VI)/Mo(V) coupling, MoO3 is of interest in electrochemical devices and displays.[6] Molybdenum trioxide has also been suggested as a potential anti-microbial agent, e.g., in polymers. In contact with water, it forms H+ ions that can kill bacteria effectively.[7] However, the issue of keeping the catalyst clean in an environment that would exploit such antimicrobial properties has not been explored.

References
- 1 2 3 "Molybdenum (soluble compounds, as Mo)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- 1 2 Roger F. Sebenik et al. (2005). "Molybdenum and Molybdenum Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_655.
- 1 2 Molybdite Mineral Data
- ↑ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ↑ Heynes, J. B. B.; Cruywagen, J. J. (1986). "Yellow Molybdenum(VI) Oxide Dihydrate". Inorganic Syntheses. 24: 191–2. doi:10.1002/9780470132555.ch56.
- ↑ Ferreira, F. F.; Souza Cruz, T. G.; Fantini, M. C. A.; Tabacniks, M. H.; de Castro, S. C.; Morais, J.; de Siervo, A.; Landers, R.; Gorenstein, A. (2000). "Lithium insertion and electrochromism in polycrystalline molybdenum oxide films". Solid State Ionics. 136–137: 357. doi:10.1016/S0167-2738(00)00483-5.
- ↑ Zollfrank, Cordt; Gutbrod, Kai; Wechsler, Peter; Guggenbichler, Josef Peter (2012). "Antimicrobial activity of transition metal acid MoO3 prevents microbial growth on material surfaces". Materials Science and Engineering: C. 32: 47. doi:10.1016/j.msec.2011.09.010.
External links
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- U.S. Department of Health and Human Services National Toxicology Program
- International Molybdenum Association
- Los Alamos National Laboratory - Molybdenum
-oxid_Kristallstruktur.png)

