Lead dioxide
 | |
| Names | |
|---|---|
| IUPAC name
Lead(IV) oxide | |
| Other names
Plumbic oxide Plattnerite | |
| Identifiers | |
| ECHA InfoCard | 100.013.795 |
PubChem CID |
|
| RTECS number | OGO700000 |
| UN number | 1872 |
| Properties | |
| PbO2 | |
| Molar mass | 239.1988 g/mol |
| Appearance | dark-brown, black powder |
| Density | 9.38 g/cm3 |
| Melting point | 290 °C (554 °F; 563 K) decomposes |
| insoluble | |
| Solubility | soluble in acetic acid insoluble in alcohol |
Refractive index (nD) |
2.3 |
| Structure | |
| hexagonal | |
| Hazards | |
| Safety data sheet | External MSDS |
EU classification (DSD) (outdated) |
Repr. Cat. 1/3 |
| R-phrases (outdated) | R61, R20/22, R33, R62, R50/53 |
| S-phrases (outdated) | S53, S45, S60, S61 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Related compounds | |
Other cations |
Carbon dioxide Silicon dioxide Germanium dioxide Tin dioxide |
| Lead(II) oxide Lead(II,IV) oxide | |
Related compounds |
Thallium(III) oxide Bismuth(III) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lead(IV) oxide, commonly called lead dioxide or plumbic oxide or anhydrous plumbic acid[1] (sometimes wrongly called lead peroxide,) is a chemical compound with the formula PbO2. It is an oxide where lead is in an oxidation state of +4; bond type is predominantly covalent[2]. It is an odorless dark-brown crystalline powder which is nearly insoluble in water. It exists in two crystalline forms. The alpha phase has orthorhombic symmetry; it was first synthesized in 1941 and was identified in nature as a rare mineral scrutinyite in 1988. The more common tetragonal beta phase was first identified as the mineral plattnerite around 1845 and later produced synthetically. Lead dioxide is a strong oxidizing agent which is used in the manufacture of matches, pyrotechnics, dyes and other chemicals. It also has several important applications in electrochemistry, in particular in the positive plates of lead acid batteries.
Properties
Physical
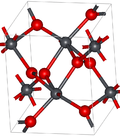

Lead dioxide is an odorless dark-brown crystalline powder which is nearly insoluble in water.[3] It has two major polymorphs, alpha and beta, which occur naturally as rare minerals scrutinyite and plattnerite, respectively. Whereas the beta form had been identified in 1845,[4] α-PbO2 was first identified in 1946 and found as a naturally occurring mineral 1988.[5]
The alpha form has orthorhombic symmetry, space group Pbcn (No. 60), Pearson symbol oP12, lattice constants a = 0.497 nm, b = 0.596 nm, c = 0.544 nm, Z = 4 (four formula units per unit cell).[5] The lead atoms are six-coordinate.
The symmetry of the beta form is tetragonal, space group P42/mnm (No. 136), Pearson symbol tP6, lattice constants a = 0.491 nm, c = 0.3385 nm, Z = 2[6] and related to the rutile structure and can be envisaged as containing columns of octahedra sharing opposite edges and joined to other chains by corners. This contrasts with the alpha form where the octahedra are linked by adjacent edges to give zigzag chains.[5]
Chemical
Lead dioxide decomposes upon heating in air as follows:
- PbO2 → Pb12O19 → Pb12O17 → Pb3O4 → PbO
The stoichiometry of the end product can be controlled by changing the temperature – for example, in the above reaction, the first step occurs at 290 °C, second at 350 °C, third at 375 °C and fourth at 600 °C. In addition, Pb2O3 can be obtained by decomposing PbO2 at 580–620 °C under an oxygen pressure of 1,400 bar (140 MPa). Therefore, thermal decomposition of lead dioxide is a common industrial way of producing various lead oxides.[7]
Lead dioxide is an amphoteric compound with prevalent acidic properties. It dissolves in strong bases to form the hydroxyplumbate ion, [Pb(OH)6]2−:[3]
- PbO2 + 2 NaOH + 2 H2O → Na2[Pb(OH)6]
It also reacts with basic oxides in the melt, yielding orthoplumbates M4[PbO4].
Because of the instability of its Pb4+ cation, lead dioxide reacts with hot acids, converting to the more stable Pb2+ state and liberating oxygen:[7]
- 2 PbO2 + 2 H2SO4 → 2 PbSO4 + 2 H2O + O2
- 2 PbO2 + 4 HNO3 → 2 Pb(NO3)2 + 2 H2O + O2
- PbO2 + 4 HCl → PbCl2 + 2 H2O + Cl2
However these reactions are slow
Lead dioxide is well known for being a good oxidizing agent, with an example reaction listed below:[8]
- 2 MnSO4 + 5 PbO2 + 6 HNO3 → 2 HMnO4 + 2 PbSO4 + 3 Pb(NO3)2 + 2 H2O
- 2 Cr(OH)3 + 10 KOH + 3 PbO2 → 2 K2CrO4 + 3K 2PbO2 + 8 H2O
Electrochemical
Although the formula of lead dioxide is nominally given as PbO2, the actual oxygen to lead ratio varies between 1.90 and 1.98 depending on the preparation method. Deficiency of oxygen (or excess of lead) results in the characteristic metallic conductivity of lead dioxide, with a resistivity as low as 10−4 Ω·cm and which is exploited in various electrochemical applications. Like metals, lead dioxide has a characteristic electrode potential, and in electrolytes it can be polarized both anodically and cathodically. Lead dioxide electrodes have a dual action, that is both the lead and oxygen ions take part in the electrochemical reactions.[9]
Production
Chemical processes
Lead dioxide is produced commercially by several methods, which include oxidation of Pb3O4 in alkaline slurry in a chlorine atmosphere,[7] reaction of lead(II) acetate with "chloride of lime" (a mixture approximating calcium hypochlorite chloride),[10] or reacting Pb3O4 with dilute nitric acid:[3][11]
- Pb3O4 + 4 HNO3 → PbO2 + 2 Pb(NO3)2 + 2 H2O
Treating PbCl2 with a sodium hypochlorite solution yields PbO2. By this way lead(II) is oxidized to lead(IV) and chlorine gas rises from the hypochlorite solution. By the decomposition of NaOCl to NaOH, stoichiometric amounts of PbO2 react with NaOH to form the hexahydroxoplumbate(IV) ion [Pb(OH)6]2−, soluble in water.
Electrolysis
An alternative synthesis method is electrochemical: lead dioxide forms on pure lead, in dilute sulfuric acid, when polarized anodically at electrode potential about +1.5 V at room temperature. This procedure is used for large-scale industrial production of PbO2 anodes. Lead and copper electrodes are immersed in sulfuric acid flowing at a rate of 5–10 L/min. The electrodeposition is carried out galvanostatically, by applying a current of about 100 A/m2 for about 30 minutes. The drawback of the lead electrode is its softness, especially compared to the hard and brittle PbO2 which has a Mohs hardness of 5.5.[12] This mismatch in mechanical properties results in peeling of the coating. Therefore, an alternative method is to use harder substrates, such as titanium, niobium, tantalum or graphite and deposit PbO2 onto them from lead(II) nitrate in static or flowing nitric acid. The substrate is usually sand-blasted before the deposition to remove surface oxide and contamination and to increase the surface roughness and adhesion of the coating.[13]
Applications
Lead dioxide is used in the production of matches, pyrotechnics, dyes and the curing of sulfide polymers. It is also used in the construction of high-voltage lightning arresters.[7]
Lead dioxide is used as an anode material in electrochemistry. Beta-PbO2 is more attractive for this purpose than the alpha form because it has relatively low resistivity, good corrosion resistance even in low-pH medium, and a high overvoltage for the evolution of oxygen in sulfuric-acid- and nitric-acid-based electrolytes. Lead dioxide can also withstand chlorine evolution in hydrochloric acid. Lead dioxide anodes are inexpensive and were once used instead of conventional platinum and graphite electrodes for regenerating potassium dichromate. They were also applied as oxygen anodes for electroplating copper and zinc in sulfate baths. In organic synthesis, lead dioxide anodes were applied for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte.[13]
The most important use of lead dioxide is as the cathode of lead acid batteries. Its utility arises from the anomalous metallic conductivity of PbO2. The lead acid battery stores and releases energy by shifting the equilibrium (a comproportionation) between metallic lead, lead dioxide, and lead(II) salts in sulfuric acid.
- Pb + PbO2 + 2 HSO−
4 + 2 H+ → 2 PbSO4 + 2 H2O E° = +2.05 V
Safety
Being a strong oxidant, lead dioxide is a poison when ingested. The associated symptoms include abdominal pain and spasms, nausea, vomiting and headache. Acute poisoning can lead to muscle weakness, metallic taste, loss of appetite, insomnia, dizziness, with shock, coma and death in extreme cases. The poisoning also results in high lead levels in blood and urine. Contact with skin or eyes results in local irritation and pain.[14]
References
- ↑ "anhydrous plumbic acid". thefreedictionary.com. Retrieved 12 April 2018.
- ↑ Meek, Terry L.; Garner, Leah D. (2005-02-01). "Electronegativity and the Bond Triangle". Journal of Chemical Education. 82 (2): 325. doi:10.1021/ed082p325. ISSN 0021-9584.
- 1 2 3 Eagleson, Mary (1994). Concise Encyclopedia of Chemistry. Walter de Gruyter. p. 590. ISBN 3-11-011451-8.
- ↑ Haidinger, W. (1845). "Zweite Klasse: Geogenide. II. Ordnung. Baryte VII. Bleibaryt. Plattnerit.". Handbuch der Bestimmenden Mineralogie (PDF) (in German). Vienna: Braumüller & Seidel. p. 500.
- 1 2 3 Taggard, J. E., Jr.; et al. (1988). "Scrutinyite, natural occurrence of α-PbO2 from Bingham, New Mexico, U.S.A., and Mapimi, Mexico" (PDF). Canadian Mineralogist. 26: 905.
- ↑ Harada, H.; Sasa, Y.; Uda, M. (1981). "Crystal data for β-PbO2". Journal of Applied Crystallography. 14 (2): 141. doi:10.1107/S0021889881008959.
- 1 2 3 4 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 386. ISBN 0-08-037941-9.
- ↑ Kumar De, Anil (2007). A Textbook of Inorganic Chemistry. New Age International. p. 387. ISBN 81-224-1384-6.
- ↑ Barak, M. (1980). Electrochemical power sources: primary and secondary batteries. IET. pp. 184 ff. ISBN 0-906048-26-5.
- ↑ Wiberg, Nils (2007). Lehrbuch der Anorganischen Chemie [Textbook of Inorganic chemistry] (in German). Berlin: de Gruyter. p. 919. ISBN 978-3-11-017770-1.
- ↑ Sutcliffe, Arthur (1930). Practical Chemistry for Advanced Students (1949 ed.). London: John Murray.
- ↑ "Plattnerite: Plattnerite mineral information and data". www.mindat.org. Retrieved 12 April 2018.
- 1 2 François Cardarelli (2008). Materials Handbook: A Concise Desktop Reference. Springer. p. 574. ISBN 1-84628-668-9.
- ↑ "LEAD DIOXIDE". hazard.com. Retrieved 12 April 2018.
