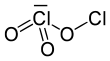
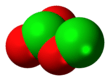
Dichlorine trioxide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
dichlorine trioxide | |||
| Other names
chlorine trioxide chlorine chlorate chlorine(I,V) oxide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| Cl2O3 | |||
| Molar mass | 118.903 g/mol | ||
| Appearance | dark brown solid | ||
| Melting point | explodes below 0 °C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Dichlorine trioxide, Cl2O3, is a chlorine oxide. It is a dark brown solid discovered in 1967 which is explosive even below 0 °C.[2] It is formed by the low-temperature photolysis of ClO2 and is formed along with Cl2O6, Cl2 and O2. Its structure is believed to be OCl−ClO2 with possible isomers such as Cl−O−ClO2.[3] It is the theoretical anhydride of chlorous acid.
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–51. ISBN 0-8493-0594-2.
- ↑ N. N. Greenwood and A. Earnshaw, (1997). Chemistry of the Elements. Butterworth-Heinemann. ISBN 978-0750633659.
- ↑ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.