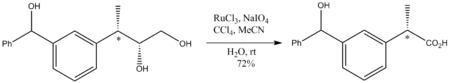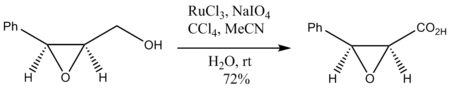Ruthenium tetroxide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ruthenium(VIII) oxide | |||
| Identifiers | |||
| ECHA InfoCard | 100.039.815 | ||
PubChem CID |
|||
| Properties | |||
| RuO4 | |||
| Molar mass | 165.07 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | pungent | ||
| Density | 3.29 g/cm3 | ||
| Melting point | 25.4 °C (77.7 °F; 298.5 K) | ||
| Boiling point | 40.0 °C (104.0 °F; 313.1 K) | ||
| 2% w/v at 20 °C | |||
| Solubility in other solvents | Soluble in Carbon tetrachloride Chloroform | ||
| Structure | |||
| tetrahedral | |||
| zero | |||
| Hazards | |||
| Safety data sheet | external MSDS sheet | ||
| NFPA 704 | |||
| Related compounds | |||
Related compounds |
RuO2 RuCl3 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Ruthenium tetroxide (Ruthenium(VIII) oxide) is the inorganic compound with the formula RuO4. It is a colourless, diamagnetic liquid, but samples are typically black due to impurities. It is volatile. The analogous OsO4 is more widely used and better known. One of the few solvents in which it forms stable solutions is CCl4.
Preparation
RuO4 is prepared by oxidation of ruthenium(III) chloride with NaIO4.
- 8 Ru3+(aq) + 5 IO4−(aq) + 12 H2O(l) → 8 RuO4(s) + 5 I−(aq) + 24 H+(aq)
Due to the expense, toxicity, and high reactivity of RuO4, it is often generated in situ and used in catalytic quantities in organic reactions, by using a substoichiometric amount of ruthenium(III) or -(IV) precursor and a stoichiometric amount of sodium metaperiodate as the terminal oxidant to continuously regenerate small amounts of RuO4. In typical reactions featuring RuO4 as the oxidant, many forms of ruthenium usefully serve as precursors to RuO4, most commonly used are RuCl3·xH2O or RuO2·xH2O.
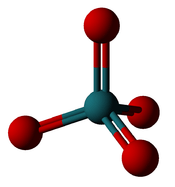
Structure
The molecule adopts a tetrahedral geometry, with the Ru–O distances ranging from 169 to 170 pm.[1]
Uses
Isolation of ruthenium from ores
The main value of RuO4 is as an intermediate in the production of ruthenium compounds and metal from ores. Like other platinum group metals (PGMs), ruthenium occurs at low concentrations and often mixed with other PGMs. Together with OsO4, it is separated from other PGMs by distillation of a chlorine-oxidized extract. Ruthenium is separated from OsO4 by reducing RuO4 with hydrochloric acid, a process that exploits the highly positive reduction potential for the [RuO4]0/- couple.[2]
Organic chemistry
RuO4 is only of specialized value in organic chemistry because it oxidizes virtually any hydrocarbon. For example, it will oxidize adamantane to 1-adamantanol. Because it is such an aggressive oxidant, reaction conditions must be mild, generally room temperature. Although a strong oxidant, RuO4 oxidations do not perturb stereocenters that are not oxidized. Illustrative is the oxidation of the following diol to a carboxylic acid:
Oxidation of epoxy alcohols also occurs without degradation of the epoxide ring:
Under milder condition, oxidative reaction yields aldehydes instead. RuO4 readily converts secondary alcohols into ketones. Although similar results can be achieved with other cheaper oxidants such as PCC- or DMSO-based oxidants, RuO4 is ideal when a very vigorous oxidant is needed but mild conditions must be maintained. It is used in organic synthesis to oxidize internal alkynes to 1,2-diketones, and terminal alkynes along with primary alcohols to carboxylic acids. When used in this fashion, the ruthenium(VIII) oxide is used in catalytic amounts and regenerated by the addition of sodium periodate to ruthenium(III) chloride and a solvent mixture of acetonitrile, water and carbon tetrachloride. RuO4 readily cleaves double bonds to yield carbonyl products, in a manner similar to ozonolysis. Osmium(VIII) oxide, a more familiar oxidant that is structurally similar to RuO4, does not cleave double bonds, instead producing vicinal diol products. However, with short reaction times and carefully controlled conditions, RuO4 can also be used for dihydroxylation.[3]
Because RuO4 degrades the "double bonds" of arenes (especially electron-rich ones) by dihydroxylation and cleavage of the C-C bond in a way few other reagents can, it is useful as a "deprotection" reagent for carboxylic acids that are masked as aryl groups (typically phenyl or p-methoxyphenyl). Because the fragments formed are themselves readily oxidizable by RuO4, a substantial fraction of the arene carbon atoms undergo exhaustive oxidation to form carbon dioxide. Consequently, multiple equivalents of the terminal oxidant (often in excess of 10 equivalents per aryl ring) are required to achieve complete conversion to the carboxylic acid, limiting the practicality of the transformation.[4][5][6][7]

Although used as a direct oxidant, due to the relatively high cost of RuO4 it is also used catalytically with a cooxidant. For an oxidation of cyclic alcohols with RuO4 as a catalyst and bromate as a base, RuO4 is first activated by hydroxide:
- RuO4 + OH− → HRuO5−
The reaction proceeds via a glycolate complex.
Other uses
Ruthenium tetroxide is a potential staining agent. It is used to expose latent fingerprints by turning to the brown/black ruthenium dioxide when in contact with fatty oils or fats contained in sebaceous contaminants of the print.[8]
Related ruthenium compounds
Because RuO4 will readily decompose explosively at slightly elevated temperatures, most laboratories do not synthesize it directly, nor is it commercially available. A related reagent is the anionic Ru(VII) derivative in the form of the salt of "TPAP" (tetrapropylammonium perruthenate), [N(C3H7)4]RuO4. TPAP is synthesized by oxidizing RuCl3 to RuO4− by NaBrO3 and isolated as the tetrapropylamine cation, which allows the salt to be used in organic solvents.
References
- ↑ Pley, M.; Wickleder, M. S. (2005). "Two Crystalline Modifications of RuO4". Journal of Solid State Chemistry. 178 (10): 3206–3209. doi:10.1016/j.jssc.2005.07.021.
- ↑ Bernardis, F. L.; Grant, R. A.; Sherrington, D. C. "A review of methods of separation of the platinum-group metals through their chloro-complexes" Reactive and Functional Polymers 2005, Vol. 65,, p. 205-217. doi:10.1016/j.reactfunctpolym.2005.05.011
- ↑ Plietker, Bernd; Niggemann, Meike (2003-09). "An Improved Protocol for the RuO4-Catalyzed Dihydroxylation of Olefins". Organic Letters. 5 (18): 3353–3356. doi:10.1021/ol035335a. ISSN 1523-7060. Check date values in:
|date=(help) - ↑ Nunez, M. Teresa; Martin, Victor S. (1990-03). "Efficient oxidation of phenyl groups to carboxylic acids with ruthenium tetraoxide. A simple synthesis of (R)-.gamma.-caprolactone, the pheromone of Trogoderma granarium". The Journal of Organic Chemistry. 55 (6): 1928–1932. doi:10.1021/jo00293a044. ISSN 0022-3263. Check date values in:
|date=(help) - ↑ Nasr, Khaled; Pannier, Nadine; Frangioni, John V.; Maison, Wolfgang (2008-02). "Rigid Multivalent Scaffolds Based on Adamantane". The Journal of Organic Chemistry. 73 (3): 1056–1060. doi:10.1021/jo702310g. ISSN 0022-3263. PMC 2505186. PMID 18179237. Check date values in:
|date=(help) - ↑ "Oxidative degradation of benzene rings". Tetrahedron. 59 (8): 1105–1136. 2003-02-17. doi:10.1016/S0040-4020(02)01492-8. ISSN 0040-4020.
- ↑ Martín, Victor S.; Palazón, José M.; Rodríguez, Carmen M.; Nevill, C. Richard; Hutchinson, Douglas K. (2013-09-16), "Ruthenium(VIII) Oxide", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, doi:10.1002/047084289x.rr009.pub3, ISBN 0471936235, retrieved 2018-06-20
- ↑ Mashiko, K.; Miyamoto, T. (1998). "Latent Fingerprint Processing by the Ruthenium Tetroxide Method". Journal of Forensic Identification. 48 (3): 279–290. doi:10.3408/jasti.2.21.
Further reading
- Cotton, S.A. (1997). Chemistry of Precious Metals. London: Chapman and Hall. ISBN 978-0-7514-0413-5.
- Martín, V. S.; Palazón, J. M.; Rodríguez, C. M.; Nevill, C. R. (2006). "Ruthenium(VIII) Oxide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rr009.pub2. ISBN 0471936235.
- Farmer, V.; Welton, T. (2002). "The oxidation of alcohols in substituted imidazolium ionic liquids using ruthenium catalysts". Green Chemistry. 4 (2): 97. doi:10.1039/B109851A.
- Singh, B.; Srivastava, S. (1991). "Kinetics and mechanism of ruthenium tetroxide catalysed oxidation of cyclic alcohols by bromate in a base". Transition Metal Chemistry. 16 (4): 466. doi:10.1007/BF01129466.
- Courtney, J.L.; Swansbor, K.F. (1972). "Ruthenium tetroxide oxidation". Reviews of Pure and Applied Chemistry. 22: 47.