Cofilin 1
Cofilin 1 (non-muscle; n-cofilin), also known as CFL1, is a human gene, part of the ADF/cofilin family.
Cofilin is a widely distributed intracellular actin-modulating protein that binds and depolymerizes filamentous F-actin and inhibits the polymerization of monomeric G-actin in a pH-dependent manner. It is involved in the translocation of actin-cofilin complex from cytoplasm to nucleus.[4]
One group reports that reelin signaling leads to serine3-phosphorylation of cofilin-1, and this interaction may play a role in the reelin-related regulation of neuronal migration.[5][6]
References
- GRCh38: Ensembl release 89: ENSG00000172757 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: CFL1 cofilin 1 (non-muscle)".
- Chai X, Förster E, Zhao S, Bock HH, Frotscher M (January 2009). "Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3". J. Neurosci. 29 (1): 288–99. doi:10.1523/JNEUROSCI.2934-08.2009. PMC 6664910. PMID 19129405.
- Frotscher M, Chai X, Bock HH, Haas CA, Förster E, Zhao S (April 2009). "Role of Reelin in the development and maintenance of cortical lamination". J Neural Transm. 116 (11): 1451–5. doi:10.1007/s00702-009-0228-7. PMID 19396394.
- Saito Y, Doi K, Yamagishi N, Ishihara K, Hatayama T (Feb 2004). "Screening of Hsp105alpha-binding proteins using yeast and bacterial two-hybrid systems". Biochem. Biophys. Res. Commun. 314 (2): 396–402. doi:10.1016/j.bbrc.2003.12.108. PMID 14733918.
- Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massague J, Bernard O, Soosairaiah J (Sep 2003). "Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1". J. Cell Biol. 162 (6): 1089–98. doi:10.1083/jcb.200212060. PMC 2172847. PMID 12963706.
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S (Aug 1999). "Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase". Science. 285 (5429): 895–8. doi:10.1126/science.285.5429.895. PMID 10436159.
Further reading
- Maciver SK, Hussey PJ (2002). "The ADF/cofilin family: actin-remodeling proteins". Genome Biol. 3 (5): reviews3007. doi:10.1186/gb-2002-3-5-reviews3007. PMC 139363. PMID 12049672.
- Samstag Y, Nebl G (2004). "Interaction of cofilin with the serine phosphatases PP1 and PP2A in normal and neoplastic human T lymphocytes". Adv. Enzyme Regul. 43: 197–211. doi:10.1016/S0065-2571(02)00031-6. PMID 12791392.
- Ogawa K, Tashima M, Yumoto Y, Okuda T, Sawada H, Okuma M, Maruyama Y (1991). "Coding sequence of human placenta cofilin cDNA". Nucleic Acids Res. 18 (23): 7169. doi:10.1093/nar/18.23.7169. PMC 332815. PMID 2263493.
- van der Steege G, Draaijers TG, Grootscholten PM, Osinga J, Anzevino R, Velonà I, Den Dunnen JT, Scheffer H, Brahe C, van Ommen GJ (1995). "A provisional transcript map of the spinal muscular atrophy (SMA) critical region". Eur. J. Hum. Genet. 3 (2): 87–95. doi:10.1159/000472281. PMID 7552146.
- Davidson MM, Haslam RJ (1994). "Dephosphorylation of cofilin in stimulated platelets: roles for a GTP-binding protein and Ca2+". Biochem. J. 301 ( Pt 1) (Pt 1): 41–7. doi:10.1042/bj3010041. PMC 1137140. PMID 8037689.
- Ono S, Minami N, Abe H, Obinata T (1994). "Characterization of a novel cofilin isoform that is predominantly expressed in mammalian skeletal muscle". J. Biol. Chem. 269 (21): 15280–6. PMID 8195165.
- Abe H, Nagaoka R, Obinata T (1993). "Cytoplasmic localization and nuclear transport of cofilin in cultured myotubes". Exp. Cell Res. 206 (1): 1–10. doi:10.1006/excr.1993.1113. PMID 8482351.
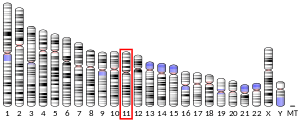
- Gillett GT, Fox MF, Rowe PS, Casimir CM, Povey S (1996). "Mapping of human non-muscle type cofilin (CFL1) to chromosome 11q13 and muscle-type cofilin (CFL2) to chromosome 14". Ann. Hum. Genet. 60 (Pt 3): 201–11. doi:10.1111/j.1469-1809.1996.tb00423.x. PMID 8800436.
- Okada K, Takano-Ohmuro H, Obinata T, Abe H (1996). "Dephosphorylation of cofilin in polymorphonuclear leukocytes derived from peripheral blood". Exp. Cell Res. 227 (1): 116–22. doi:10.1006/excr.1996.0256. PMID 8806458.
- Nebl G, Meuer SC, Samstag Y (1996). "Dephosphorylation of serine 3 regulates nuclear translocation of cofilin". J. Biol. Chem. 271 (42): 26276–80. doi:10.1074/jbc.271.42.26276. PMID 8824278.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Ott DE, Coren LV, Kane BP, Busch LK, Johnson DG, Sowder RC, Chertova EN, Arthur LO, Henderson LE (1996). "Cytoskeletal proteins inside human immunodeficiency virus type 1 virions". J. Virol. 70 (11): 7734–43. PMC 190843. PMID 8892894.
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K (1998). "Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization". Nature. 393 (6687): 809–12. Bibcode:1998Natur.393..809Y. doi:10.1038/31735. PMID 9655398.
- Rodal AA, Tetreault JW, Lappalainen P, Drubin DG, Amberg DC (1999). "Aip1p Interacts with Cofilin to Disassemble Actin Filaments". J. Cell Biol. 145 (6): 1251–64. doi:10.1083/jcb.145.6.1251. PMC 2133144. PMID 10366597.
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S (1999). "Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase". Science. 285 (5429): 895–8. doi:10.1126/science.285.5429.895. PMID 10436159.
- Sumi T, Matsumoto K, Takai Y, Nakamura T (2000). "Cofilin Phosphorylation and Actin Cytoskeletal Dynamics Regulated by Rho- and Cdc42-Activated Lim-Kinase 2". J. Cell Biol. 147 (7): 1519–32. doi:10.1083/jcb.147.7.1519. PMC 2174243. PMID 10613909.
- Adachi R, Matsui S, Kinoshita M, Nagaishi K, Sasaki H, Kasahara T, Suzuki K (2001). "Nitric oxide induces chemotaxis of neutrophil-like HL-60 cells and translocation of cofilin to plasma membranes". Int. J. Immunopharmacol. 22 (11): 855–64. doi:10.1016/S0192-0561(00)00045-X. PMID 11090694.
- Lee K, Jung J, Kim M, Guidotti G (2001). "Interaction of the alpha subunit of Na,K-ATPase with cofilin". Biochem. J. 353 (Pt 2): 377–85. doi:10.1042/0264-6021:3530377. PMC 1221581. PMID 11139403.
- Toshima J, Toshima JY, Amano T, Yang N, Narumiya S, Mizuno K (2001). "Cofilin Phosphorylation by Protein Kinase Testicular Protein Kinase 1 and Its Role in Integrin-mediated Actin Reorganization and Focal Adhesion Formation". Mol. Biol. Cell. 12 (4): 1131–45. doi:10.1091/mbc.12.4.1131. PMC 32292. PMID 11294912.
- Sumi T, Matsumoto K, Shibuya A, Nakamura T (2001). "Activation of LIM kinases by myotonic dystrophy kinase-related Cdc42-binding kinase alpha". J. Biol. Chem. 276 (25): 23092–6. doi:10.1074/jbc.C100196200. PMID 11340065.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.