Ciramadol
Ciramadol (WY-15,705) is an opioid analgesic that was developed in the late 1970s[1] and is related to phencyclidine, tramadol, tapentadol and venlafaxine.[2] It is a mixed agonist-antagonist for the μ-opioid receptor with relatively low abuse potential[3] and a ceiling on respiratory depression[4] which makes it a relatively safe drug. It has a slightly higher potency and effectiveness as an analgesic than codeine,[5] but is weaker than morphine.[6] Other side effects include sedation and nausea but these are generally less severe than with other similar drugs.[7]
 | |
| Clinical data | |
|---|---|
| Other names | Ciramadol, WY-15705 |
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
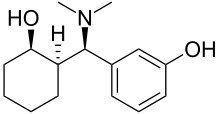
| Formula | C15H23NO2 |
| Molar mass | 249.354 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
References
- US Patent 3928626 - Benzylamine Analgesics
- Cochrane AD, Bell R, Sullivan JR, Shaw J. Ciramadol. A new analgesic. Medical Journal of Australia. 1979 Nov 3;2(9):501-2.
- Preston KL, Bigelow GE, Liebson IA. Comparative evaluation of morphine, pentazocine and ciramadol in postaddicts. Journal of Pharmacology and Experimental Therapeutics. 1987 Mar;240(3):900-10.
- Romagnoli A, Keats AS. Low ceiling respiratory depression by ciramadol. International Journal of Clinical Pharmacology Research. 1986;6(6):451-5.
- Downing JW, Brock-Utne JG, Holloway AM. Ciramadol - a new synthetic analgesic. A double-blind comparison with oral codeine for postoperative pain relief. South African Medical Journal. 1983 Dec 10;64(25):978-82.
- Powell WF. A double-blind comparison of multiple intramuscular doses of ciramadol, morphine, and placebo for the treatment of postoperative pain. Anesthesia and Analgesia. 1985 Nov;64(11):1101-7.
- Stambaugh JE Jr, McAdams J. Comparison of the analgesic efficacy and safety oral ciramadol, codeine, and placebo in patients with chronic cancer pain. Journal of Clinical Pharmacology. 1987 Feb;27(2):162-6.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.