Bromine trifluoride
Bromine trifluoride is an interhalogen compound with the formula BrF3. It is a straw-coloured liquid with a pungent odor.[5] It is soluble in sulfuric acid but reacts violently with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.[6]
 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.211 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 1746 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| BrF3 | |
| Molar mass | 136.90 g/mol |
| Appearance | straw-coloured liquid hygroscopic |
| Odor | Choking, pungent[1] |
| Density | 2.803 g/cm3 [2] |
| Melting point | 8.77 °C (47.79 °F; 281.92 K) |
| Boiling point | 125.72 °C (258.30 °F; 398.87 K) |
| Reacts with water[3] | |
| Solubility in sulfuric acid | very soluble |
| Structure | |
| T-shaped (C2v) | |
| 1.19 D | |
| Hazards[4] | |
| Main hazards | dangerously sensitive to water, source of HF |
| Safety data sheet | See: data page http://www.chammascutters.com/en/downloads/Bromine-Trifluoride-MSDS.pdf |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H271, H330, H314, H373 |
| P102, P103, P210, P220, P221, P260, P264, P271, P280, P283, P284, P301+310, P301+330+331, P303+361+353, P304+312, P306+360, P308+313, P370+380, P340, P363, P305+351+338+310 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
Bromine monochloride |
Other cations |
Chlorine trifluoride Iodine trifluoride |
Related compounds |
Bromine monofluoride Bromine pentafluoride |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Bromine trifluoride was first described by Paul Lebeau in 1906, who obtained the material by the reaction of bromine with fluorine at 20 °C:[7]
- Br2 + 3 F2 → 2 BrF3
The disproportionation of bromine monofluoride also gives bromine trifluoride:[5]
- 3 BrF → BrF3 + Br2
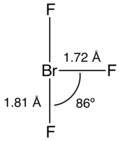
Structure
Like ClF3 and IF3, the BrF3 molecule is T-shaped and planar. In the VSEPR formalism, the bromine center is assigned two electron pairs. The distance from the bromine each axial fluorine is 1.81 Å and to the equatorial fluorine is 1.72 Å. The angle between an axial fluorine and the equatorial fluorine is slightly smaller than 90° — the 86.2° angle observed is due to the repulsion generated by the electron pairs being greater than that of the Br-F bonds.[8][9]
Chemical properties
BrF3 rapidly and exothermically reacts with water to release hydrobromic acid and hydrofluoric acid:
- BrF3 + 2H2O → 3HF + HBr + O2
BrF3 is a fluorinating agent, but less reactive than ClF3. The liquid is conducting, owing to autoionisation:[6]
- 2 BrF3 ⇌ BrF2+ + BrF4−
Many ionic fluorides dissolve readily in BrF3 forming fluoroanions:[6]
- KF + BrF3 → KBrF4
Covalent fluorides may also react as fluoride acceptor, which make them acidic in this solvent:[10]
- BrF3 + SbF5 → [BrF2+][SbF6−]
References
- http://www.chammascutters.com/en/downloads/Bromine-Trifluoride-MSDS.pdf
- Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- "Archived copy" (PDF). Archived from the original (PDF) on 2012-05-13. Retrieved 2012-11-25.CS1 maint: archived copy as title (link)
- "Safety Data Sheet Bromine Trifluoride" (PDF). Airgas. Retrieved 16 January 2020.
- Simons JH (1950). "Bromine(III) Fluoride (Bromine Trifluoride)". Bromine (III) Fluoride - Bromine Trifluoride. Inorganic Syntheses. 3. pp. 184–186. doi:10.1002/9780470132340.ch48. ISBN 978-0-470-13234-0.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Lebeau P. (1906). "The effect of fluorine on chloride and on bromine". Annales de Chimie et de Physique. 9: 241–263.
- Gutmann V (1950). "Die Chemie in Bromitrifluorid". Angewandte Chemie. 62 (13–14): 312–315. doi:10.1002/ange.19500621305.
- Meinert H (1967). "Interhalogenverbindungen". Zeitschrift für Chemie. 7 (2): 41–57. doi:10.1002/zfch.19670070202.
- A. J. EDWARDS and G. R. JONES. JChern. Soc. (London) A, 1467 (1969)
