Lithium hexafluorophosphate
Lithium hexafluorophosphate is an inorganic compound with the formula LiPF6. It is a white crystalline powder. It is used in commercial secondary batteries, an application that exploits its high solubility in non aqueous, polar solvents. Specifically, solutions of lithium hexafluorophosphate in carbonate blends of ethylene carbonate, dimethyl carbonate, diethyl carbonate and/or ethyl methyl carbonate, with a small amount of one or many additives such as fluoroethylene carbonate and vinylene carbonate, serve as state-of-the-art electrolytes in lithium-ion batteries.[1] [2] This application also exploits the inertness of the hexafluorophosphate anion toward strong reducing agents, such as lithium metal.
 | |
| Names | |
|---|---|
| IUPAC name
lithium hexafluorophosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.040.289 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| LiPF6 | |
| Molar mass | 151.905 g/mol |
| Appearance | white powder |
| Density | 1.5 g/cm3 |
| Melting point | 200 °C (392 °F; 473 K) |
| soluble | |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms | 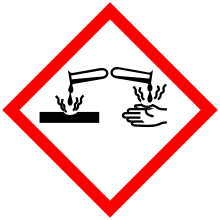 |
| GHS Signal word | Danger |
GHS hazard statements |
H314 |
| P280, P310, P305+351+338 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Lithium tetrafluoroborate |
Other cations |
Sodium hexafluorophosphate Potassium hexafluorophosphate Ammonium hexafluorophosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The salt is relatively stable thermally, but loses 50% weight at 200 °C (392 °F). It hydrolyzes near 70 °C (158 °F)[3] according to the following equation forming highly toxic HF gas:
- LiPF6 + H2O → HF + PF5 + LiOH
Owing to the Lewis acidity of the Li+ ions, LiPF6 also catalyses the tetrahydropyranylation of tertiary alcohols.[4]
In lithium-ion batteries, LiPF6 reacts with Li2CO3, which may be catalysed by small amounts of HF: [5]
- LiPF6 + Li2CO3 → POF3 + CO2 + 3 LiF
A recent research also proposed, based on nuclear magnetic resonance results and density functional theory calculations, that LiPF6 could react in a mixture of ethylene carbonate and dimethyl carbonate with alumina powder or alumina coated lithium-ion batteries cathode materials to form lithium difluorophosphate and alumina oxyfluoride via the reaction: [6]
- 2 Al2O3 (s) + x LiPF6 (solv) → 2 Al2O3-xF2x (s) + x LiPO2F2 (solv)
Since lithium difluorophosphate is known to improve the performance of lithium-ion batteries, this suggest a new mechanism of action for alumina coatings in lithium-ion batteries.
References
- Goodenough, John B.; Kim, Youngsik (9 February 2010). "Challenges for Rechargeable Li Batteries". Chemistry of Materials. 22 (3): 587–603. doi:10.1021/cm901452z.
- Qian, Yunxian; Hu, Shiguang; Zou, Xianshuai; Deng, Zhaohui; Xu, Yuqun; Cao, Zongze; Kang, Yuanyuan; Deng, Yuanfu; Shi, Qiao; Xu, Kang; Deng, Yonghong (2019). "How electrolyte additives work in Li-ion batteries". Energy Storage Materials. 20: 208–215. doi:10.1016/j.ensm.2018.11.015. ISSN 2405-8297.
- Xu, Kang (October 2004). "Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries". Chemical Reviews. 104 (10): 4303–4418. doi:10.1021/cr030203g. PMID 15669157.
- Nao Hamada; Sato Tsuneo (2004). "Lithium Hexafluorophosphate-Catalyzed Efficient Tetrahydropyranylation of Tertiary Alcohols under Mild Reaction Conditions". Synlett (10): 1802–1804. doi:10.1055/s-2004-829550.
- Bi, Yujing; Wang, Tao; Liu, Meng; Du, Rui; Yang, Wenchao; Liu, Zixuan; Peng, Zhe; Liu, Yang; Wang, Deyu; Sun, Xueliang (2016). "Stability of Li2CO3 in cathode of lithium ion battery and its influence on electrochemical performance". RSC Advances. 6 (23): 19233–19237. doi:10.1039/C6RA00648E. ISSN 2046-2069.
- Hall, David S.; Gauthier, Roby; Eldesoky, Ahmed; Murray, Vivian S.; Dahn, J.R. (2019-04-17). "New Chemical Insights into the Beneficial Role of Al 2 O 3 Cathode Coatings in Lithium-ion Cells". ACS Applied Materials & Interfaces. 11 (15): 14095–14100. doi:10.1021/acsami.8b22743. ISSN 1944-8244.