Phosphorus pentabromide
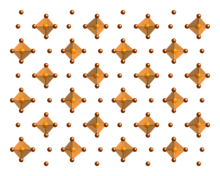
Phosphorus pentabromide is a reactive, yellow solid of formula PBr5, which has the structure PBr4+ Br− in the solid state but in the vapor phase is completely dissociated to PBr3 and Br2. Rapid cooling of this phase to 15 K leads to formation of the ionic species phosphorus heptabromide ([PBr4]+[Br3]−).[1]
 | |
_bromide.jpg) | |
| Names | |
|---|---|
| IUPAC name
phosphorus pentabromide | |
| Other names
phosphorus(V) bromide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.260 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| PBr5 | |
| Molar mass | 430.49 g/mol |
| Appearance | yellow solid |
| Density | 3.61 g/cm3 |
| Melting point | ca. 100 °C (decomposes) |
| Boiling point | 106 °C (223 °F; 379 K) (decomposes) |
| decomposes | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It can be used in organic chemistry to convert carboxylic acids to acyl bromides. It is highly corrosive. It decomposes above 100 °C to give phosphorus tribromide and bromine:[2]
Reversing this equilibrium to generate PBr5 by addition of Br2 to PBr3 is difficult in practice because the product is susceptible to further addition to yield phosphorus heptabromide (PBr7).[3]
References
- https://books.google.com.my/books?id=5ufh9rM5ko0C&pg=PA154&lpg=PA154&dq=rapid+cooling+of+pbr5&source=bl&ots=7T1GFWzxFg&sig=ACfU3U27MrYXojqE6faSgeWLLAq-5dKS7Q&hl=zh-CN&sa=X&ved=2ahUKEwiJz4CAuKvpAhUTheYKHQZsD_YQ6AEwDHoECAgQAQ#v=onepage&q=rapid%20cooling%20of%20pbr5&f=false
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Popov, A. I.; Skelly, N. E. (1954). "Spectrophotometric Study of Phosphorus Pentabromide in Various Solvents". J. Am. Chem. Soc. 76 (15): 3916–3919. doi:10.1021/ja01644a014.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.