Palladium(II) bromide
Palladium(II) bromide is an inorganic compound of palladium and bromine with the chemical formula PdBr2. It is a commercially available, though less common than palladium(II) chloride, the usual entry point to palladium chemistry. Unlike the chloride, palladium(II) bromide is insoluble in water, but dissolves when heated in acetonitrile to give monomeric acetonitrile adducts:[1]
- PdBr2 + 2 MeCN → PdBr2(MeCN)2
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.248 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Br2Pd | |
| Molar mass | 266.228 g/mol |
| Related compounds | |
Other anions |
Palladium(II) fluoride Palladium(II) chloride Palladium(II) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
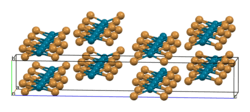
The structure of PdBr2 has been determined by X-ray crystallography.[2] It crystallises in the P21/c space group and the structure consists of wavy ribbons of edge-sharing PdBr4 coordination squares.[3]


References
- O. A. Zalevskaya, E. G. Vorob’eva1, I. A. Dvornikova and A. V. Kuchin (2008). "Palladium complexes based on optically active terpene derivatives of ethylenediamine". Russian Journal of Coordination Chemistry. 34 (11): 855–857. doi:10.1134/S1070328408110110.CS1 maint: multiple names: authors list (link)
- K. Brodersen G. Thiele H. Gaedcke (1966). "Die Konstitution des Palladium(II)‐bromids". Z. Anorg. Allg. Chem. 348 (3–4): 162–167. doi:10.1002/zaac.19663480307.
- "Information card for entry 1534319". Crystallography Open Database. Retrieved 2020-05-03.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.