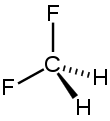
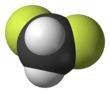
Difluoromethane
Difluoromethane, also called HFC-32 or R-32, is an organic compound of the dihalogenoalkane variety. It has the formula of CH2F2.
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Difluoromethane[1] | |||
| Other names
Carbon fluoride hydride Methylene difluoride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | HFC-32 R-32 | ||
| 1730795 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.764 | ||
| EC Number |
| ||
| 259463 | |||
| MeSH | Difluoromethane | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3252 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CH2F2 | |||
| Molar mass | 52.024 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 1.1 g cm−3(in liquid form) | ||
| Melting point | −136 °C (−213 °F; 137 K) | ||
| Boiling point | −52 °C (−62 °F; 221 K) | ||
| log P | -0.611 | ||
| Vapor pressure | 1518.92 kPa (at 21.1 °C) | ||
| Hazards | |||
| Safety data sheet | See: data page MSDS at Oxford University | ||
| R-phrases (outdated) | R11 | ||
| S-phrases (outdated) | S9, S16, S33 | ||
| NFPA 704 (fire diamond) | |||
| 648 °C (1,198 °F; 921 K) | |||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Uses
Difluoromethane is a molecule used as refrigerant that has zero ozone depletion potential (ODP) [2], a global warming potential (GWP) index 675 times that of carbon dioxide, based on a 100-year time frame [3], and it is classified as A2L - slightly flammable by ASHRAE.[4]
Difluoromethane has excellent heat transfer and pressure drop performance both in condensation and vaporisation. Therefore, in spite of its flammability rating, it seems to be a very promising low GWP substitute for traditional HFC refrigerants. [5]
Difluoromethane in a zeotropic (50/50 mass%) mixture with pentafluoroethane (R-125) is known as R-410A, a common replacement for various chlorofluorocarbons (aka Freon) in new refrigerant systems, especially for air-conditioning. The zeotropic mix of difluoromethane with pentafluoroethane (R-125) and tetrafluoroethane (R-134a) is known as R-407A through R-407E depending on the composition. Likewise the azeotropic (48.2/51.8 mass%) mixture with chlorotrifluoromethane (R13).
Difluoromethane is currently used in residential and commercial air-conditioners in Japan, China, and India as a substitute for R-410A. In order to reduce the residual risk associated with its mild flammability, this molecule should be applied in heat transfer equipment with low refrigerant charge such as brazed plate heat exchangers (BPHE), or shell and tube heat exchangers and tube and plate heat exchangers with tube of small diameter [6]. Many applications confirmed that difluoromethane exhibits heat transfer coefficients higher than those of R-410A under the same operating conditions but also higher frictional pressure drops [7].
References
- "Difluoromethane - Compound Summary". The PubChem Project. USA: National Center of Biotechnological Information.
- "R32".
- May 2010 TEAP XXI/9 Task Force Report
- 2009 ASHRAE Handbook
- Longo, Giovanni A.; Mancin, Simone; Righetti, Giulia; Zilio, Claudio (2015). "HFC32 vaporisation inside a Brazed Plate Heat Exchanger (BPHE): Experimental measurements and IR thermography analysis". International Journal of Refrigeration. 57: 77–86. doi:10.1016/j.ijrefrig.2015.04.017.
- Longo, Giovanni A.; Mancin, Simone; Righetti, Giulia; Zilio, Claudio (2016). "HFC32 and HFC410A flow boiling inside a 4 mm horizontal smooth tube". International Journal of Refrigeration. 61: 12–22. doi:10.1016/j.ijrefrig.2015.09.002.
- Longo, Giovanni A.; Mancin, Simone; Righetti, Giulia; Zilio, Claudio (2016). "HFC32 and HFC410A flow boiling inside a 4 mm horizontal smooth tube". International Journal of Refrigeration. 61: 12–22. doi:10.1016/j.ijrefrig.2015.09.002.
External links
- Flammability Measurements of Difluoromethane in Air at 100 °C
- Difluoromethane at Gas Encyclopaedia
- IR absorption spectra