Axomadol
Axomadol (INN, USAN) (code name EN3324) is a synthetic, centrally-acting opioid analgesic of the benzenoid class which was under investigation by Endo Pharmaceuticals in collaboration with Grünenthal for the treatment of chronic, moderate to severe lower back pain and arthrosis.[1][2] Development was halted after phase II clinical trials as it did not meet the pre-determined clinical endpoints.[3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
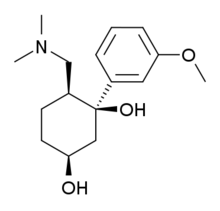
| Formula | C16H25NO3 |
| Molar mass | 279.380 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Chadds Ford (2011-06-30). "Endo Announces Topline Results From Phase 2 Study of Axomadol in Chronic Low Back Pain" (Press release). Endo Pharmaceuticals. Retrieved 2012-05-11.
- US Abandoned 20100331424, Schiene K, Bloms-Funke P, Bothmer J, Lefeber C, "Use of Axomadol for Treatment of Arthrosis Pain", published 2010-12-30, assigned to Grünenthal GmbH
- Chadds Ford (2011-08-17). "Endo Pharmaceuticals Announces Termination of Collaboration with Grunenthal for the Development of Axomadol" (Press release). Endo Pharmaceuticals. Retrieved 2012-05-11.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.