Organocobalt chemistry

Organocobalt chemistry is the chemistry of organometallic compounds containing a carbon to cobalt chemical bond. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12 has a cobalt-carbon bond. Many organocobalt compounds exhibit useful catalytic properties, the preeminent example being dicobalt octacarbonyl.[1]
Carbonyl complexes
Dicobalt octacarbonyl reacts with hydrogen and alkenes to give aldehydes. This reaction is the basis of hydroformylation, the formation of aldehydes from an alkene, CO and hydrogen. A key intermediate is cobalt tetracarbonyl hydride (HCo(CO)4). The original Ruhrchemie process produced propanal from ethene and syngas using cobalt carbonyl has been displaced by rhodium-based catalysts. Processes involving cobalt are practiced by BASF, EXXON, and Shell mainly for the production of C7-C14 alcohols used for the production of surfactants.[2]
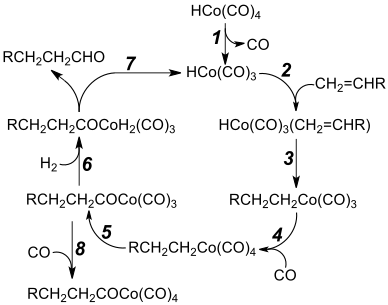
In hydrocarboxylations hydrogen is replaced by water or an alcohol and the reaction product is a carboxylic acid or an ester. An example of this reaction type is the conversion of butadiene to adipic acid. Cobalt catalysts (together with iron) are relevant in the Fischer-Tropsch process in which it is assumed that organocobalt intermediates form.
Alkyne derivatives of Co2(CO)8
Dicobalt octacarbonyl also reacts with alkynes to give "tetrahedranes" of the formula Co2(CO)6(C2R2). Because the cobalt carbonyl centers can be removed later, it functions as a protective group for the alkyne. In the Nicholas reaction an alkyne group is also protected and at the same time the alpha-carbon position is activated for nucleophilic substitution.
Cyclization reactions
Cobalt compounds react with dialkynes and dienes to cyclic intermediates in cyclometalation. Other alkynes, alkenes, nitriles or carbon monoxide can then insert themselves into the Co-C bond. Reaction types based on this concept are the Pauson–Khand reaction (CO insertion) and alkyne trimerization (notably with cyclopentadienylcobalt dicarbonyl).
Cp, allyl, and alkene compounds
Sandwich compounds
Organocobalt compounds are known with alkene, allyl, diene, and Cp ligands. A famous sandwich compound is cobaltocene, a 19-electron metallocene that is used as a reducing agent and a source of CpCo. Other sandwich compounds are CoCp(C6Me6) and Co(C6Me6)2, with 20 electrons and 21 electrons, respectively. Reduction of anhydrous cobalt(II) chloride with sodium in the presence of cyclooctadiene gives Co(cyclooctadiene)(cyclooctenyl), a synthetically versatile reagent.[5]
(C8H13).png)
CpCo(CO)2 and derivatives
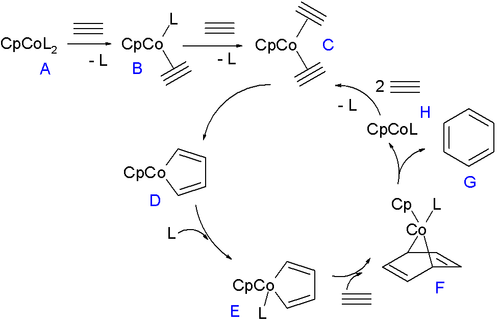
The half-sandwich compounds are particularly versatile catalysts. These include cyclopentadienylcobalt dicarbonyl (CpCo(CO)2) as well as the olefin complexes CpCo(C2H4)2 and CpCo(cod). They catalyze alkyne trimerisation.[6] which has been applied to the synthesis of a variety of complex structures.[7]
Vitamin B12-type compounds
Cobalt is found in vitamin B12 and related enzymes. These cofactors catalyze unusual reactions involving the intermediacy of Co-C bonds. In these reactions, the oxidation state of cobalt can vary from Co(III) to Co(I). In methylcobalamin the ligand is a methyl group, which is electrophilic. in vitamin B12, the alkyl ligand is an adenosyl group. Related to vitamin B12 are cobalt porphyrins, dimethylglyoximates, and related complexes of Schiff base ligands. These synthetic compounds also form alkyl derivatives that undergo diverse reactions reminiscent of the biological processes. The weak cobalt(III)-carbon bond in vitamin B12 analogues can be exploited in a type of Cobalt mediated radical polymerization of acrylic and vinyl esters (e.g. vinyl acetate), acrylic acid and acrylonitrile.[8]
Miscellaneous
An early example of organocobalt chemistry is the carbonylation of azobenzene with dicobalt octacarbonyl as described by Murahashi & Horiie in 1956:[9]
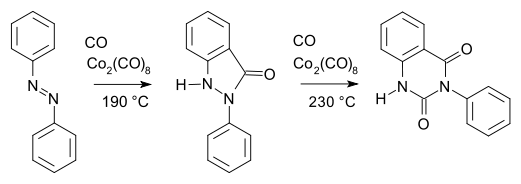
See also
- Chemical bonds of carbon with other elements in the periodic table:
| Compounds of carbon with other elements in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ↑ Omae, Iwao (2007). "Three characteristic reactions of organocobalt compounds in organic synthesis". Applied Organometallic Chemistry. 21 (5): 318. doi:10.1002/aoc.1213.
- ↑ Boy Cornils, Wolfgang A. Herrmann, Chi-Huey Wong, Horst Werner Zanthoff: Catalysis from A to Z: A Concise Encyclopedia, 2408 Seiten, Verlag Wiley-VCH Verlag GmbH & Co. KGaA, (2012), ISBN 3-527-33307-X.
- ↑ Richard F. Heck, David S. Breslow (1961). "The Reaction of Cobalt Hydrotetracarbonyl with Olefins". Journal of the American Chemical Society. 83: 4023–4027. doi:10.1021/ja01480a017. .
- ↑ Jack Halpern (2001). "'Organometallic chemistry at the threshold of a new millennium. Retrospect and prospect". Pure and Applied Chemistry. 73: 209–220.
- ↑ Gosser, L. W.; Cushing, M. A., Jr. (1977). "π-Cyclooctenyl-π-1,5-cyclooctadienecobalt". Inorganic Syntheses. 17: 112–15. doi:10.1002/9780470132487.ch32.
- ↑ Cobalt-Catalyzed Cyclotrimerization of Alkynes: The Answer to the Puzzle of Parallel Reaction Pathways Nicolas Agenet, Vincent Gandon, K. Peter C. Vollhardt, Max Malacria, Corinne Aubert J. Am. Chem. Soc.; 2007; 129(28) pp 8860 - 8871; (Article) doi:10.1021/ja072208r
- ↑ Chebny VJ, Dhar D, Lindeman SV, Rathore R (2006). "Simultaneous Ejection of Six Electrons at a Constant Potential by Hexakis(4-ferrocenylphenyl)benzene". Org. Lett. 8 (22): 5041–5044. doi:10.1021/ol061904d. PMID 17048838.
- ↑ Antoine, Debuigne; Poli, Rinaldo; Jérôme, Christine; Jérôme, Robert; Detrembleur, Christophe (2009). "Overview of cobalt-mediated radical polymerization: Roots, state of the art and future prospects". Progress in Polymer Science. 34 (3): 211–239. doi:10.1016/j.progpolymsci.2008.11.003.
- ↑ Murahashi, Shunsuke; Horiie, Shigeki (1956). "The reaction of azobenzene and carbon monoxide". Journal of the American Chemical Society. 78 (18): 4816. doi:10.1021/ja01599a079.