Alkyne trimerisation
An alkyne trimerisation reaction is a 2+2+2 cyclization reaction in which three molecules of alkyne react to form an arene. The reaction requires metal catalysts. The process is of historic interest as well as being applicable to organic synthesis.[1] Many variations have been developed including cyclization of mixtures of alkynes and alkenes as well as alkynes and nitriles.
History
In 1948, Reppe discovered that nickel compounds catalyze the formation of substituted benzenes from acetylenes:[2][3]
- 3 RC2H → C6R3H3
Both 1,3,5- and 1,2,4-C6R3H3 isomers are observed.
Since Reppe's discovery, many other cyclotrimerizations have been reported.[4][5][6][7][8][9]
Mechanism and stereochemistry
The reaction does not occur without the assistance of metal catalysis. The reactions begin with the formation of metal-alkyne complexes. The combination of two alkynes within the coordination sphere affords a metallacyclopentadiene.[10] Starting from the metallacyclopentadiene intermediate, two pathways are usually discussed:
- (1) the third alkyne insertion generates metallocycloheptatriene. Reductive elimination directly from a metallocycloheptatriene to form an arene is symmetry forbidden,[11] but stepwise mechanisms have been demonstrated.
- (2) the third alkyne participates in [4+2] cycloaddition to generate a bicyclic species (a metallanorbornadiene).
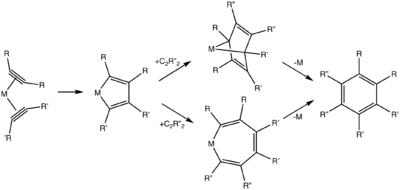
For cyclotrimerization reactions of unsymmetrically substituted acetylenes, the substitution pattern about the product arene is determined in two steps: formation of the metallocyclopentadiene intermediate and incorporation of the third equivalent of alkyne. Although both head-to-head and tail-to-tail metallocyclopentadienes lead to 1, a number of acetylenic substrates selectively form regioisomers of type 2. Steric bulk on the alkyne coupling partners and catalyst have been invoked as the controlling elements of regioselectivity.[12]
Chiral catalysts have been employed in combination with arynes to produce non-racemic atropisomeric products.[13]
Scope and limitations
Catalysts for cyclotrimerization are highly selective for triple bonds, which gives the reaction a fairly wide substrate scope; alcohols, ethers, amines, and carbonyl compounds (ketones, esters, amides, and carboxylic acids) are all well tolerated. Nitriles can react with alkynes to give pyridines.[14]
In addition, some reactions are limited by catalyst deactivation via formation of stable, 18-electron η4-complexes.[15] Cyclobutadiene, cyclohexadiene, and arene complexes have all been observed as off-cycle, inactivate catalyst forms. In addition to high-order polymers and dimers and trimers, which originate from low regio- and chemoselectivities, enyne side products derived from alkyne dimerization have been observed. Rhodium catalysts are particularly adept at enyne formation (see below).[16] For nickel catalysis, formation of larger rings (particularly cyclooctatetraene) can be a problem.
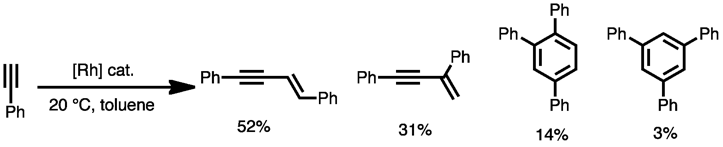
Synthetic applications
Cyclization of diynes with a separate alkyne can provide fused ring systems with high atom economy. In a striking example in the synthesis of estrone, the diyne reactant shown below was combined with di(trimethylsilyl)acetylene to produce the benzocyclobutene product indicated[17] Upon heating, ring-opening produced a quinone methide that participated in a subsequent intramolecular Diels-Alder reaction.
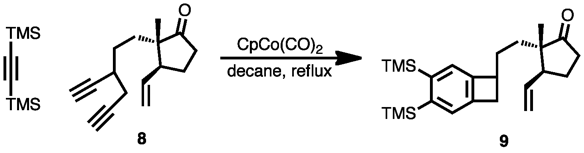
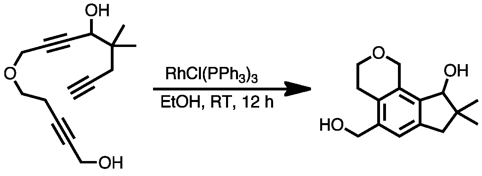
If the third alkyne unit is tethered to the first two, three rings can be created in a single step. In the example below in the synthesis of calomelanolactone, Wilkinson's catalyst was used to catalyze an intramolecular cotrimerization of the triyne shown.[18]
Crowded triynes can cyclize to products exhibiting helical chirality. In one example remarkable for the formation of three new aromatic rings in one step, the triyne shown is transformed into the helical product via treatment with cyclopentadienylcobalt dicarbonyl.[19] As of 2004, this process had yet to be rendered asymmetric, but the products could be separated through chiral HPLC.[19]
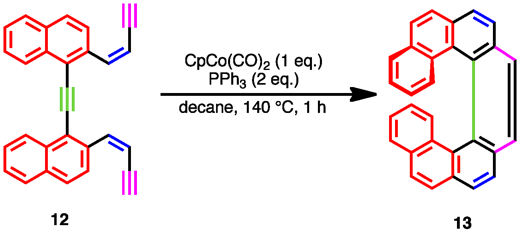
Comparison with other methods
Cyclotrimerization presents an alternative to the functionalization of pre-formed aromatic compounds through electrophilic or nucleophilic substitution, the regioselectivity of which can sometimes be difficult to control.
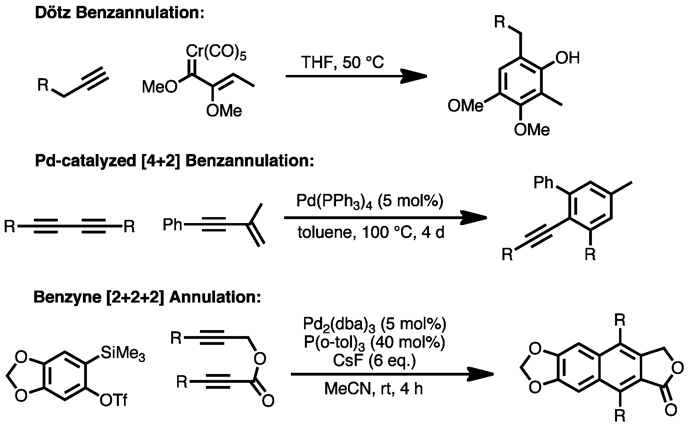
Other methods for the direct formation of aromatic rings from substituted, unsaturated precursors include the Dötz reaction, palladium-catalyzed [4+2] benzannulation of enynes with alkynes,[20] and Lewis-acid-mediated [4+2] cycloaddition of enynes with alkynes.[21] Cyclization of transient benzyne species with alkynes, catalyzed by palladium, can also produce substituted aromatic compounds.[22]
References
- ↑ Agenet, N.; Buisine, O.; Slowinski, F.; Gandon, V.; Aubert, C.; Malacria, M. (2007). "Cotrimerizations of Acetylenic Compounds". Org. React. 68: 1–302. doi:10.1002/0471264180.or068.01. ISBN 0471264180.
- ↑ Reppe, W.; Schweckendiek, W. J. (1948). "Cyclisierende Polymerisation von Acetylen. III Benzol, Benzolderivate und hydroaromatische Verbindungen". Liebigs Ann. Chem. 560: 104–116. doi:10.1002/jlac.19485600104.
- ↑ Reppe, W.; Vetter, H. (1953). "Carbonylierung VI. Synthesen mit Metallcarbonylwasserstoffen". Liebigs Ann. Chem. 585: 133. doi:10.1002/jlac.19535820107.
- ↑ Musso, F.; Solari, E.; Floriani, C. (1997). "Hydrocarbon Activation with Metal Halides: Zirconium Tetrachloride Catalyzing the Jacobsen Reaction and Assisting the Trimerization of Alkynes via the Formation of η6-Arene−Zirconium(IV) Complexes". Organometallics. 16 (22): 4889. doi:10.1021/om970438g.
- ↑ Rodríguez, J. Gonzalo; Martín-Villamil, Rosa; Fonseca, Isabel (1997). "Tris(2,4-pentanedionato)vanadium-catalysed cyclotrimerization and polymerization of 4-(N,N-dimethylamino)phenylethyne: X-ray structure of 1,2,4-tris[4-(N,N -dimethylamino)phenyl]benzene". Journal of the Chemical Society, Perkin Transactions 1 (6): 945–948. doi:10.1039/a605474i. ISSN 0300-922X.
- ↑ Sakurai, H.; Nakadaira, Y.; Hosomi, A.; Eriyama, Y.; Hirama, K.; Kabuto, C. (1984). "Chemistry of organosilicon compounds. 193. Intramolecular cyclotrimerization of macrocylic and acyclic triynes with Group 6 metal carbonyls. The formation of fulvene and benzene". J. Am. Chem. Soc. 106 (26): 8315. doi:10.1021/ja00338a063.
- ↑ Amer, I.; Bernstein, T.; Eisen, M.; Blum, J.; Vollhardt, K. P. C. (1990). "Oligomerization of alkynes by the RhCl3-aliquat 336 catalyst system Part 1. Formation of benzene derivatives". J. Mol. Catal. 60 (3): 313. doi:10.1016/0304-5102(90)85254-F.
- ↑ Lee, C. L.; Hunt, C. T.; Balch, A. L. (1981). "Novel reactions of metal-metal bonds. Reactions of Pd2{(C6H5)2PCH2P(C6H5)2}2Cl2 with acetylenes, olefins, and isothiocyanates". Inorg. Chem. 20 (8): 2498. doi:10.1021/ic50222a026.
- ↑ Aalten, H. L.; van Koten, G.; Riethorst, E.; Stam, C. H. (1989). "The Hurtley reaction. 2. Novel complexes of disubstituted acetylenes with copper(I) benzoates having a reactive ortho carbon-chlorine or carbon-bromine bond. X-ray structural characterization of tetrakis(2-chlorobenzoato)bis(diethyl acetylenedicarboxylate)tetracopper(I)". Inorg. Chem. 28 (22): 4140. doi:10.1021/ic00321a020.
- 1 2 Ma, Wangyang; Yu, Chao; Chen, Tianyang; Xu, Ling; Zhang, Wen-Xiong; Xi, Zhenfeng (2017). "Metallacyclopentadienes: synthesis, structure and reactivity". Chemical Society Reviews. 46 (4): 1160–1192. doi:10.1039/C6CS00525J. ISSN 0306-0012.
- ↑ Hardesty, J. H.; Koerner, J. B.; Albright, T. A.; Lee, G. B. (1999). "Theoretical Study of the Acetylene Trimerization with CpCo". J. Am. Chem. Soc. 121 (25): 6055. doi:10.1021/ja983098e.
- ↑ Ozerov, O. V.; Patrick, B. O.; Ladipo, F. T. (2000). "Highly Regioselective [2 + 2 + 2] Cycloaddition of Terminal Alkynes Catalyzed by η6-Arene Complexes of Titanium Supported by Dimethylsilyl-Bridgedp-tert-Butyl Calix[4]arene Ligand". J. Am. Chem. Soc. 122 (27): 6423. doi:10.1021/ja994543o.
- ↑ Shibata, Takanori; Tsuchikama, Kyoji (2008). "Recent advances in enantioselective [2 + 2 + 2] cycloaddition". Organic & Biomolecular Chemistry. 6 (8): 1317. doi:10.1039/b720031e. ISSN 1477-0520.
- ↑ Varela, Jesus; Saa, Carlos (March 20, 2003). "Construction of Pyridine Rings by Metal-Mediated [2 + 2 + 2] Cycloaddition†". Chemistry Review. 103: 3787–3802. doi:10.1021/cr030677f. Retrieved April 11, 2015.
- ↑ Kölle, U.; Fuss, B. (1986). "Pentamethylcyclopentadienyl-Übergangsmetall-Komplexe, X. Neue Co-Komplexe aus η5-C5Me5Co-Fragmenten und Acetylenen". Chem. Ber. 119: 116. doi:10.1002/cber.19861190112.
- ↑ Ardizzoia, G. A.; Brenna, S.; Cenini, S.; LaMonica, G.; Masciocchi, N.; Maspero, A. (2003). "Oligomerization and Polymerization of Alkynes Catalyzed by Rhodium(I) Pyrazolate Complexes". J. Mol. Catal. A: Chemical. 204–205: 333–340. doi:10.1016/S1381-1169(03)00315-7.
- ↑ Vollhardt, K. Peter C. (1984). "Cobalt-assisted [2+2+2] cycloadditions: a synthesis strategy grows to maturity". Angewandte Chemie. 96: 525–41. doi:10.1002/ange.19840960804.
- ↑ Neeson, S. J.; Stevenson, P. J. (1988). "Rhodium catalysed [2+2+2] cycloadditions- an efficient regiospecific route to calomelanolactone". Tetrahedron Lett. 29 (7): 813. doi:10.1016/S0040-4039(00)80217-8.
- 1 2 Teply, F.; Stara, I. G.; Stary, I.; Kollarovic, A.; Saman, D.; Rulisek, L.; Fiedler, P. (2002). "Synthesis of 5-, 6-, and 7helicene via Ni(0)- or Co(I)-catalyzed isomerization of aromatic cis,cis-dienetriynes". J. Am. Chem. Soc. 124 (31): 9175–80. doi:10.1021/ja0259584. PMID 12149022.
- ↑ Gevorgyan, V.; Takeda, A.; Homma, M.; Sadayori, N.; Radhakrishnan, U.; Yamamoto, Y. (1999). "Palladium-Catalyzed [4+2]Cross-Benzannulation Reaction of Conjugated Enynes with Diynes and Triynes". J. Am. Chem. Soc. 121 (27): 6391. doi:10.1021/ja990749d.
- ↑ Wills, M. S. B.; Danheiser, R. L. (1998). "Intramolecular [4 + 2] Cycloaddition Reactions of Conjugated Ynones. Formation of Polycyclic Furans via the Generation and Rearrangement of Strained Heterocyclic Allenes". J. Am. Chem. Soc. 120 (36): 9378. doi:10.1021/ja9819209.
- ↑ Sato, Y.; Tamura, T.; Mori, M. (2004). "Arylnaphthalene lignans through Pd-Catalyzed 2+2+2 cocyclization of arynes and diynes: total synthesis of Taiwanins C and E". Angew. Chem. Int. Ed. Engl. 43 (18): 2436–40. doi:10.1002/anie.200453809. PMID 15114584.