Surfactant
Surfactants are compounds that lower the surface tension (or interfacial tension) between two liquids, between a gas and a liquid, or between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, and dispersants.
Etymology and definition
The term surfactant is a blend of surface active agent.[1]
In the United States National Library of Medicine's Medical Subject Headings (MeSH) vocabulary, surfactant is reserved for the meaning pulmonary surfactant. For the more general meaning, surface active agent/s is the heading.

Composition and structure
Surfactants are usually organic compounds that are amphiphilic, meaning they contain both hydrophobic groups (their tails) and hydrophilic groups (their heads).[2] Therefore, a surfactant contains both a water-insoluble (or oil-soluble) component and a water-soluble component. Surfactants will diffuse in water and adsorb at interfaces between air and water or at the interface between oil and water, in the case where water is mixed with oil. The water-insoluble hydrophobic group may extend out of the bulk water phase, into the air or into the oil phase, while the water-soluble head group remains in the water phase.
World production of surfactants is estimated at 15 Mton/y, of which about half are soaps. Other surfactants produced on a particularly large scale are linear alkylbenzene sulfonates (1700 kton/y), lignin sulfonates (600 kton/y), fatty alcohol ethoxylates (700 ktons/y), and alkylphenol ethoxylates (500 kton/y).[3]
 Sodium stearate, the most common component of most soap, which comprises about 50% of commercial surfactants.
Sodium stearate, the most common component of most soap, which comprises about 50% of commercial surfactants.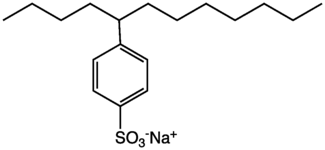 4-(5-Dodecyl) benzenesulfonate, a linear dodecylbenzenesulfonate, one of the most common surfactants.
4-(5-Dodecyl) benzenesulfonate, a linear dodecylbenzenesulfonate, one of the most common surfactants.
Structure of surfactant phases in water
In the bulk aqueous phase, surfactants form aggregates, such as micelles, where the hydrophobic tails form the core of the aggregate and the hydrophilic heads are in contact with the surrounding liquid. Other types of aggregates can also be formed, such as spherical or cylindrical micelles or lipid bilayers. The shape of the aggregates depends on the chemical structure of the surfactants, namely the balance in size between the hydrophilic head and hydrophobic tail. A measure of this is the HLB, Hydrophilic-lipophilic balance. Surfactants reduce the surface tension of water by adsorbing at the liquid-air interface. The relation that links the surface tension and the surface excess is known as the Gibbs isotherm.
Dynamics of surfactants at interfaces
The dynamics of surfactant adsorption is of great importance for practical applications such as in foaming, emulsifying or coating processes, where bubbles or drops are rapidly generated and need to be stabilized. The dynamics of adsorption depend on the diffusion coefficient of the surfactant. As the interface is created, the adsorption is limited by the diffusion of the surfactant to the interface. In some cases, there can exist an energetic barrier to adsorption or desorption of the surfactant. If such a barrier limits the adsorption rate, the dynamics are said to be ‘kinetically limited'. Such energy barriers can be due to steric or electrostatic repulsions. The surface rheology of surfactant layers, including the elasticity and viscosity of the layer, play an important role in the stability of foams and emulsions.
Characterization of interfaces and surfactant layers
Interfacial and surface tension can be characterized by classical methods such as the -pendant or spinning drop method. Dynamic surface tensions, i.e. surface tension as a function of time, can be obtained by the maximum bubble pressure apparatus
The structure of surfactant layers can be studied by ellipsometry or X-Ray reflectivity.
Surface rheology can be characterized by the oscillating drop method or shear surface rheometers such as double-cone, double-ring or magnetic rod shear surface rheometer.
Detergents in biochemistry and biotechnology
In solution, detergents help solubilize a variety of chemical species by dissociating aggregates and unfolding proteins. Popular surfactants in the biochemistry laboratory are SDS and CTAB. Detergents are key reagents to extract protein by lysis of the cells and tissues: They disorganize the membrane's lipidic bilayer (SDS, Triton X-100, X-114, CHAPS, DOC, and NP-40), and solubilize proteins. Milder detergents such as octyl thioglucoside, octyl glucoside or dodecyl maltoside are used to solubilize membrane proteins such as enzymes and receptors without denaturing them. Non-solubilized material is harvested by centrifugation or other means. For electrophoresis, for example, proteins are classically treated with SDS to denature the native tertiary and quaternary structures, allowing the separation of proteins according to their molecular weight.
Detergents have also been used to decellularise organs. This process maintains a matrix of proteins that preserves the structure of the organ and often the microvascular network. The process has been successfully used to prepare organs such as the liver and heart for transplant in rats.[4] Pulmonary surfactants are also naturally secreted by type II cells of the lung alveoli in mammals.
Classification
The "tails" of most surfactants are fairly similar, consisting of a hydrocarbon chain, which can be branched, linear, or aromatic. Fluorosurfactants have fluorocarbon chains. Siloxane surfactants have siloxane chains.
Many important surfactants include a polyether chain terminating in a highly polar anionic group. The polyether groups often comprise ethoxylated (polyethylene oxide-like) sequences inserted to increase the hydrophilic character of a surfactant. Polypropylene oxides conversely, may be inserted to increase the lipophilic character of a surfactant.
Surfactant molecules have either one tail or two; those with two tails are said to be double-chained.

Most commonly, surfactants are classified according to polar head group. A non-ionic surfactant has no charged groups in its head. The head of an ionic surfactant carries a net positive, or negative charge. If the charge is negative, the surfactant is more specifically called anionic; if the charge is positive, it is called cationic. If a surfactant contains a head with two oppositely charged groups, it is termed zwitterionic. Commonly encountered surfactants of each type include:
Anionic
Sulfate, sulfonate, and phosphate esters
Anionic surfactants contain anionic functional groups at their head, such as sulfate, sulfonate, phosphate, and carboxylates. Prominent alkyl sulfates include ammonium lauryl sulfate, sodium lauryl sulfate (sodium dodecyl sulfate, SLS, or SDS), and the related alkyl-ether sulfates sodium laureth sulfate (sodium lauryl ether sulfate or SLES), and sodium myreth sulfate.
Others include:
- Docusate (dioctyl sodium sulfosuccinate)
- Perfluorooctanesulfonate (PFOS)
- Perfluorobutanesulfonate
- Alkyl-aryl ether phosphates
- Alkyl ether phosphates
Carboxylates
These are the most common surfactants and comprise the carboxylate salts (soaps), such as sodium stearate. More specialized species include sodium lauroyl sarcosinate and carboxylate-based fluorosurfactants such as perfluorononanoate, perfluorooctanoate (PFOA or PFO).
Cationic head groups
- pH-dependent primary, secondary, or tertiary amines: Primary and secondary amines become positively charged at pH < 10:"Bordwell pKa Table (Acidity in DMSO)". Retrieved 11 May 2014.
- Permanently charged quaternary ammonium salts:
Zwitterionic surfactants
Zwitterionic (amphoteric) surfactants have both cationic and anionic centers attached to the same molecule. The cationic part is based on primary, secondary, or tertiary amines or quaternary ammonium cations. The anionic part can be more variable and include sulfonates, as in the sultaines CHAPS (3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate) and cocamidopropyl hydroxysultaine. Betaines such as cocamidopropyl betaine have a carboxylate with the ammonium. The most common biological zwitterionic surfactants have a phosphate anion with an amine or ammonium, such as the phospholipids phosphatidylserine, phosphatidylethanolamine, phosphatidylcholine, and sphingomyelins.
Nonionic
Nonionic surfactants have covalently bonded oxygen-containing hydrophilic groups, which are bonded to hydrophobic parent structures. The water-solubility of the oxygen groups is the result of hydrogen bonding. Hydrogen bonding decreases with increasing temperature, and the water solubility of nonionic surfactants therefore decreases with increasing temperature.
Nonionic surfactants are less sensitive to water hardness than anionic surfactants, and they foam less strongly. The differences between the individual types of nonionic surfactants are slight, and the choice is primarily governed having regard to the costs of special properties (e.g., effectiveness and efficiency, toxicity, dermatological compatibility, biodegradability) or permission for use in food.[3]
Ethoxylates
Fatty alcohol ethoxylates
Alkylphenol ethoxylates
Ethoxylated amines and/or fatty acid amides
Terminally blocked ethoxylates
Fatty acid esters of polyhydroxy compounds
Fatty acid esters of glycerol
Fatty acid esters of sorbitol
Alkyl polyglucosides
Amine oxides
Sulfoxides
Phosphine oxides
According to the composition of their counter-ion
In the case of ionic surfactants, the counter-ion can be:
- Monatomic/Inorganic:
- Cations: metals : alkali metal, alkaline earth metal, transition metal
- Anions: halides: chloride (Cl−), bromide (Br−), iodide (I−)
- Polyatomic / Organic:
- Cations: ammonium, pyridinium, triethanolamine (TEA)
- Anions: tosyls, trifluoromethanesulfonates, methyl sulfate
In pharmacy
A wetting agent is a surfactant that, when dissolved in water, lowers the advancing contact angle, aids in displacing an air phase at the surface, and replaces it with a liquid phase. Examples of application of wetting to pharmacy and medicine include the displacement of air from the surface of sulfur, charcoal, and other powders for the purpose of dispersing these drugs in liquid vehicles; the displacement of air from the matrix of cotton pads and bandages so that medicinal solutions can be absorbed for application to various body areas; the displacement of dirt and debris by the use of detergents in the washing of wounds; and the application of medicinal lotions and sprays to surface of skin and mucous membranes.
Pharmaceutical forms
The human body produces different types of surfactant in different parts or organs for different purposes. Pulmonary surfactant is produced in lungs in order to facilitate breathing by increasing total lung capacity, TLC, and lung compliance. In respiratory distress syndrome or RDS surfactant replacement therapy helps patients have normal respiration by using pharmaceutical forms of the surfactants. One of the most important pharmaceutical pulmonary surfactants is Survanta (beractant) or its generic form Beraksurf produced by Abbvie and Tekzima respectively.
Current market and forecast
The annual global production of surfactants was 13 million tonnes in 2008.[5][6] In 2014, the world market for surfactants reached a volume of more than 33 billion US-dollars. Market researchers expect annual revenues to increase by 2.5% per year to around 40.4 billion US-dollars until 2022. The commercially most significant type of surfactants is currently the anionic surfactant alkyl benzene sulfonate (LAS), which is widely used in cleaners and detergents.[7]
Health and environmental controversy
Surfactants are routinely deposited in numerous ways on land and into water systems, whether as part of an intended process or as industrial and household waste. Some of them are known to be toxic to animals, ecosystems, and humans, and can increase the diffusion of other environmental contaminants.[8][9][10] As a result, there are proposed or voluntary restrictions on the use of some surfactants. For example, PFOS is a persistent organic pollutant as judged by the Stockholm Convention. Additionally, PFOA has been subject to a voluntary agreement by the U.S. Environmental Protection Agency and eight chemical companies to reduce and eliminate emissions of the chemical and its precursors.[11]
The two major surfactants used in the year 2000 were linear alkylbenzene sulfonates (LAS) and the alkyl phenol ethoxylates (APE). They break down in the aerobic conditions found in sewage treatment plants and in soil to the metabolite nonylphenol, which is thought to be an endocrine disruptor.[12][13]
Ordinary dishwashing detergent, for example, will promote water penetration in soil, but the effect would last only a few days (many standard laundry detergent powders contain levels of chemicals such as alkali and chelating agents that can be damaging to plants and should not be applied to soils). Commercial soil wetting agents will continue to work for a considerable period, but they will eventually be degraded by soil micro-organisms. Some can, however, interfere with the life-cycles of some aquatic organisms, so care should be taken to prevent run-off of these products into streams, and excess product should not be washed down.
Anionic surfactants can be found in soils as the result of sludge application, wastewater irrigation, and remediation processes. Relatively high concentrations of surfactants together with multimetals can represent an environmental risk. At low concentrations, surfactant application is unlikely to have a significant effect on trace metal mobility.[14][15]
Biosurfactants
Biosurfactants are surface-active substances synthesised by living cells. Interest in microbial surfactants is due to their diversity, environmentally friendly nature, possibility of large-scale production, selectivity, performance under extreme conditions, and potential applications in environmental protection.[16][17] A few of the popular examples of microbial biosurfactants includes Emulsan produced by Acinetobacter calcoaceticus,[18] Sophorolipids produced by several yeasts belonging to candida and the starmerella clade,[19][20] and Rhamnolipid produced by Pseudomonas aeruginosa[21] etc.
Biosurfactants enhance the emulsification of hydrocarbons, have the potential to solubilise hydrocarbon contaminants and increase their availability for microbial degradation. The use of chemicals for the treatment of a hydrocarbon polluted site may contaminate the environment with their by-products, whereas biological treatment may efficiently destroy pollutants, while being biodegradable themselves. Hence, biosurfactant-producing microorganisms may play an important role in the accelerated bioremediation of hydrocarbon-contaminated sites.[22][23][24] These compounds can also be used in enhanced oil recovery and may be considered for other potential applications in environmental protection.[24][25] Other applications include herbicides and pesticides formulations, detergents, healthcare and cosmetics, pulp and paper, coal, textiles, ceramic processing and food industries, uranium ore-processing, and mechanical dewatering of peat.[16][17][26]
Several microorganisms are known to synthesise surface-active agents; most of them are bacteria and yeasts.[27][28] When grown on hydrocarbon substrate as the carbon source, these microorganisms synthesise a wide range of chemicals with surface activity, such as glycolipid, phospholipid, and others.[29][30] These chemicals are synthesised to emulsify the hydrocarbon substrate and facilitate its transport into the cells. In some bacterial species such as Pseudomonas aeruginosa, biosurfactants are also involved in a group motility behavior called swarming motility.
Safety and environmental risks
Most anionic and nonionic surfactants are nontoxic, having LD50 comparable to sodium chloride. The situation for cationic surfactants is more diverse. Dialkyldimethylammonium chlorides have very low LD50's (5 g/kg) but alkylbenzyldimethylammonium chloride has an LD50 of 0.35 g/kg. Prolonged exposure of skin to surfactants can cause chafing because surfactants (e.g., soap) disrupts the lipid coating that protects skin (and other) cells.[3]
Biosurfactants and Deepwater Horizon
The use of biosurfactants as a way to remove petroleum from contaminated sites has been studied and found to be safe and effective in the removal of petroleum products from soil. Other studies found that surfactants are often more toxic than the oil that is being dispersed, and the combination of the oil and the surfactant can be more toxic than either alone. Biosurfactants were not used by BP after the Deepwater Horizon oil spill. However, unprecedented amounts of Corexit (active ingredient: dioctyl sodium sulfosuccinate (DOSS), sorbitan monooleate (Span 80), and polyoxyethylenated sorbitan monooleate (Tween-80)),[31][32] were sprayed directly into the ocean at the leak and on the sea-water's surface, the theory being that the surfactants isolate droplets of oil, making it easier for petroleum-consuming microbes to digest the oil.
Biosurfactants produced by microbe or bacteria can be used to enhance oil production by microbial enhanced oil recovery method (MEOR).[33]
Applications
Surfactants play an important role as cleaning, wetting, dispersing, emulsifying, foaming and anti-foaming agents in many practical applications and products, including:
- Detergents
- Fabric softeners
- Emulsions
- Soaps
- Paints
- Adhesives
- Inks
- Anti-fogs
- Ski waxes, snowboard wax
- Deinking of recycled papers, in flotation, washing and enzymatic processes
- Laxatives
- Agrochemical formulations
- Herbicides (some)
- Insecticides
- Quantum dot in order to manipulate growth[34] and assembly of the dots, reactions on their surface, electrical properties, etc., it is important to understand how surfactants arrange[35] on the surface of the quantum dots
- Biocides (sanitizers)
- Cosmetics:
- Shampoos
- Shower gel
- Hair conditioners (after shampoo)
- Toothpastes
- Spermicides (nonoxynol-9)
- Firefighting
- Pipelines, liquid drag reducing agent
- Alkali Surfactant Polymers (used to mobilize oil in oil wells)[36]
- Ferrofluids
- Leak Detectors
- Natural Insecticide against flying insects such as Honey Bees
- Plasticizer in Nanocellulose[37]
See also
- Anti-fog
- Cleavable detergent
- Emulsion
- Hydrotrope
- MBAS assay, an assay that indicates anionic surfactants in water with a bluing reaction.
- Niosome
- Oil dispersants
- Surfactants in paint
References
- ↑ Rosen MJ & Kunjappu JT (2012). Surfactants and Interfacial Phenomena (4th ed.). Hoboken, New Jersey: John Wiley & Sons. p. 1. ISBN 978-1-118-22902-6. Archived from the original on 8 January 2017.
- ↑ "Bubbles, Bubbles, Everywhere, But Not a Drop to Drink". The Lipid Chronicles. 11 November 2011. Archived from the original on 26 April 2012. Retrieved 1 August 2012.
- 1 2 3 Kurt Kosswig "Surfactants" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2005, Weinheim. doi:10.1002/14356007.a25_747
- ↑ Wein, Harrison (28 June 2010). "Progress Toward an Artificial Liver Transplant – NIH Research Matters". National Institutes of Health (NIH). Archived from the original on 5 August 2012.
- ↑ "Market Report: World Surfactant Market". Acmite Market Intelligence. Archived from the original on 13 September 2010.
- ↑ Reznik, Gabriel O.; Vishwanath, Prashanth; Pynn, Michelle A.; Sitnik, Joy M.; Todd, Jeffrey J.; Wu, Jun; Jiang, Yan; Keenan, Brendan G.; et al. (2010). "Use of sustainable chemistry to produce an acyl amino acid surfactant". Applied Microbiology and Biotechnology. 86 (5): 1387–97. doi:10.1007/s00253-009-2431-8. PMID 20094712.
- ↑ Market Study on Surfactants (2nd edition, April 2015), by Ceresana Research Archived 20 March 2012 at the Wayback Machine.
- ↑ Metcalfe, Tracy L.; Dillon, Peter J.; Metcalfe, Chris D. (2008). "DETECTING THE TRANSPORT OF TOXIC PESTICIDES FROM GOLF COURSES INTO WATERSHEDS IN THE PRECAMBRIAN SHIELD REGION OF ONTARIO, CANADA". Environmental Toxicology and Chemistry. 27 (4): 811–8. doi:10.1897/07-216.1. PMID 18333674.
- ↑ Emmanuel, E; Hanna, K; Bazin, C; Keck, G; Clement, B; Perrodin, Y (2005). "Fate of glutaraldehyde in hospital wastewater and combined effects of glutaraldehyde and surfactants on aquatic organisms". Environment International. 31 (3): 399–406. doi:10.1016/j.envint.2004.08.011. PMID 15734192.
- ↑ Murphy, M; Alkhalidi, M; Crocker, J; Lee, S; Oregan, P; Acott, P (2005). "Two formulations of the industrial surfactant, Toximul, differentially reduce mouse weight gain and hepatic glycogen in vivo during early development: effects of exposure to Influenza B Virus". Chemosphere. 59 (2): 235–46. Bibcode:2005Chmsp..59..235M. doi:10.1016/j.chemosphere.2004.11.084. PMID 15722095.
- ↑ USEPA: "2010/15 PFOA Stewardship Program" Archived 27 October 2008 at the Wayback Machine. Accessed October 26, 2008.
- ↑ Mergel, Maria. "Nonylphenol and Nonylphenol Ethoxylates." Toxipedia.org. N.p., 1 Nov. 2011. Web. 27 Apr. 2014.
- ↑ Scott, M; Jones, Malcolm N (2000). "The biodegradation of surfactants in the environment". Biochimica et Biophysica Acta (BBA) – Biomembranes. 1508: 235–251. doi:10.1016/S0304-4157(00)00013-7.
- ↑ Hernández-Soriano Mdel, C; Degryse, F; Smolders, E (2011). "Mechanisms of enhanced mobilisation of trace metals by anionic surfactants in soil". Environmental Pollution (Barking, Essex : 1987). 159 (3): 809–16. doi:10.1016/j.envpol.2010.11.009. PMID 21163562.
- ↑ Hernández-Soriano Mdel, C; Peña, A; Dolores Mingorance, M (2010). "Release of metals from metal-amended soil treated with a sulfosuccinamate surfactant: effects of surfactant concentration, soil/solution ratio, and pH". Journal of Environmental Quality. 39 (4): 1298–305. doi:10.2134/jeq2009.0242. PMID 20830918.
- 1 2 Banat, I. M., Makkar, R. S., Cameotra, S. S. (2000). "Potential commercial applications of microbial surfactants". Appl. Microbiol. Biotechnol. 53 (5): 495–508. doi:10.1007/s002530051648. PMID 10855707.
- 1 2 Rahman, K. S. M., Thahira-Rahman, J., McClean, S., Marchant, R., Banat, I.M (2002). "Rhamnolipid biosurfactants production by strains of Pseudomonas aeruginosa using low cost raw materials". Biotechnol. Prog. 18 (6): 1277–1281. doi:10.1021/bp020071x. PMID 12467462.
- ↑ Shoham, Y; Rosenberg, M; Rosenberg, E (1983). "Bacterial degradation of emulsan". Appl. Environ. Microbiol. 46 (3): 573–579. PMC 239318. PMID 6688940.
- ↑ Kurtzman, C. P.; Price, N. P.; Ray, K. J.; Kuo, T. M. (2010). "Production of sophorolipid biosurfactants by multiple strains of the Starmerella (Candida) bombicola yeast clade". FEMS Microbiol Lett. 311 (2): 140–146. doi:10.1111/j.1574-6968.2010.02082.x. PMID 20738402.
- ↑ Parekh, V. J.; Pandit, A. B. (2011). "Optimization of fermentative production of sophorolipid biosurfactant by starmerella bombicola NRRL Y-17069 using response surface methodology". International Journal of Pharmacy and Biological Sciences. 1 (3): 103–116.
- ↑ Ito, S; Honda, H; Tomita, F; Suzuki, T (Dec 1971). "Rhamnolipids produced by Pseudomonas aeruginosa grown on n-paraffin (mixture of C 12 , C 13 and C 14 fractions)". J Antibiot (Tokyo). 24 (12): 855–859. doi:10.7164/antibiotics.24.855. PMID 4334639.
- ↑ Rosenberg, E., Ron, E. Z (1999). "High and low molecular mass microbial surfactants". Appl. Microbiol. Biotechnol. 52 (2): 154–162. doi:10.1007/s002530051502. PMID 10499255.
- ↑ Del ‘Arco, J. P., De Franca, F. P (2001). "Influence of oil contamination levels on hydrocarbon biodegradation in sandy sediments". Environ. Pollut. 110: 515–519.
- 1 2 Rahman, K. S. M., Banat, I.M., Thahira-Rahman, J., Thayumanavan, T., Lakshmanaperumalsamy, P (2002). "Bioremediation of gasoline contaminated soil by a bacterial consortium amended with poultry litter, coir pith and rhamnolipid biosurfactant". Bioresource Technology. 81 (1): 25–32. doi:10.1016/S0960-8524(01)00105-5. hdl:10149/58336. PMID 11710344.
- ↑ Shulga, A., Karpenko, E., Vildanova-Martsishin, R., Turovsky, A., Soltys, M (1999). "Biosurfactant enhanced remediation of oil-contaminated environments". Adsorpt. Sci. Technol. 18 (2): 171–176. doi:10.1260/0263617001493369.
- ↑ Ron, E. Z., Rosenberg, E (2001). "Natural roles of biosurfactants". Environ. Microbiol. 3 (4): 229–236. doi:10.1046/j.1462-2920.2001.00190.x. PMID 11359508.
- ↑ Banat, I. M (1995). "Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: a review". Bioresource Technology. 51: 1–12. doi:10.1016/0960-8524(94)00101-6.
- ↑ Kim, S.E., Lim, E. J., Lee, S.O., Lee , J. D., Lee, T.H (2000). "Purification and characterisation of biosurfactants from Nocardia sp. L-417". Biotechnol. Appl. Biochem. 31 (3): 249–253. doi:10.1042/BA19990111.
- ↑ Muriel, J.M., Bruque, J.M., Olias, J.M., Sanchez, A. J (1996). "Production of biosurfactants by Cladosporium resinae". Biotechnol. Lett. 18 (3): 235–240. doi:10.1007/BF00142937.
- ↑ Desai, J.D., Banat, I.M (1997). "Microbial production of surfactants and their commercial potential". Microbiol. Mol. Biol. Rev. 61 (1): 47–64. doi:10.1128/AEM.01737-15. PMC 232600. PMID 9106364.
- ↑ European Maritime Safety Agency. Manual on the Applicability of Oil Dispersants; Version 2; 2009. {{cite web |url=http://emsa.europa.eu/opr-documents/opr-manual-a-guidelines/download/1166/719/23.html |title=Archived copy |accessdate=2017-05-19 |deadurl=no |archiveurl=https://web.archive.org/web/20110705151503/http://www.emsa.europa.eu/opr-documents/opr-manual-a-guidelines/download/1166/719/23.html |archivedate=5 July 2011 |df=dmy-all }}
- ↑ Committee on Effectiveness of Oil Spill Dispersants (National Research Council Marine Board). Using Oil Spill Dispersants on the Sea. National Academy Press: Washington, D.C., 1989. http://www.nap.edu/openbook.php?record_id=736 (accessed Oct. 31, 2015)
- ↑ Hakiki, Farizal. A Critical Review of Microbial Enhanced Oil Recovery Using Artificial Sandstone Core: A Mathematical Model Archived 15 January 2016 at the Wayback Machine.. Paper IPA14-SE-119. Proceeding of The 38th IPA Conference and Exhibition, Jakarta, Indonesia, May 2014.
- ↑ Murray, C. B.; Kagan, C. R.; Bawendi, M. G. (2000). "Synthesis and Characterization of Monodisperse Nanocrystals and Close-Packed Nanocrystal Assemblies". Annual Review of Materials Research. 30 (1): 545–610. Bibcode:2000AnRMS..30..545M. doi:10.1146/annurev.matsci.30.1.545.
- ↑ Zherebetskyy D, Scheele M, Zhang Y, Bronstein N, Thompson C, Britt D, Salmeron M, Alivisatos P, Wang LW (2014). "Hydroxylation of the surface of PbS nanocrystals passivated with oleic acid". Science (Submitted manuscript). 344 (6190): 1380–1384. Bibcode:2014Sci...344.1380Z. doi:10.1126/science.1252727. PMID 24876347.
- ↑ Hakiki, F.; Maharsi, D.A.; Marhaendrajana, T. (2016). "Surfactant-Polymer Coreflood Simulation and Uncertainty Analysis Derived from Laboratory Study". Journal of Engineering and Technological Sciences. 47 (6): 706–724. doi:10.5614/j.eng.technol.sci.2015.47.6.9. Archived from the original on 20 August 2016.
- ↑ Guidetti, Giulia; Atifi, Siham; Vignolini, Silvia; Hamad, Wadood Y. (17 October 2016). "Flexible photonic cellulose nanocrystal films". Advanced Materials. 28 (45): 10042–10047. doi:10.1002/adma.201603386. PMC 5495155. PMID 27748533.
External links


