Friedreich's ataxia
| Friedreich's ataxia | |
|---|---|
| Synonyms | Spinocerebellar ataxia, Friedreich |
 | |
| Frataxin | |
| Specialty |
Neurology |
| Usual onset | 5-15 years |
Friedreich's ataxia is an autosomal recessive inherited disease that causes progressive damage to the nervous system. It manifests in initial symptoms of poor coordination such as gait disturbance; it can also lead to scoliosis, heart disease and diabetes, but does not affect cognitive function. The disease is progressive, and ultimately a wheelchair is required for mobility. Its incidence in the general population is roughly 1 in 50,000.
The particular genetic mutation (expansion of an intronic GAA triplet repeat in the FXN gene) leads to reduced expression of the mitochondrial protein frataxin. Over time this deficiency causes the aforementioned damage, as well as frequent fatigue due to effects on cellular metabolism.
The ataxia of Friedreich's ataxia results from the degeneration of nervous tissue in the spinal cord, in particular sensory neurons essential (through connections with the cerebellum) for directing muscle movement of the arms and legs. The spinal cord becomes thinner and nerve cells lose some of their myelin sheath (the insulating covering on some nerve cells that helps conduct nerve impulses).
The condition is named after the German physician Nikolaus Friedreich, who first described it in the 1860s.[1]
Signs and symptoms
Symptoms typically begin sometime between the ages of 5 to 15 years, but in Late Onset FA may occur in the 20s or 30s. Symptoms include any combination, but not necessarily all, of the following:
- Muscle weakness in the arms and legs
- Loss of coordination
- Vision impairment
- Hearing impairment
- Slurred speech
- Curvature of the spine (scoliosis)
- High plantar arches (pes cavus deformity of the foot)
- Diabetes (about 20% of people with Friedreich's ataxia develop carbohydrate intolerance and 10% develop diabetes mellitus)[2]
- Heart disorders (e.g., atrial fibrillation, and resultant tachycardia (fast heart rate) and hypertrophic cardiomyopathy)
It presents before 22 years of age with progressive staggering or stumbling gait and frequent falling. Lower extremities are more severely involved. The symptoms are slowly progressing. Long-term observation shows that many patients reach a plateau in symptoms in the patient's early adulthood. On average, after 10–15 years with the disease, patients lose the ability to stand or walk without assistance. However, disease progression is variable, and some patients may still be ambulatory decades after onset, while others require the use of a wheelchair within a few years.[3]
The following physical signs may be detected on physical examination:
- Cerebellar: nystagmus, fast saccadic eye movements, truncal ataxia, dysarthria, dysmetria.
- Lower motor neuron lesion: absent deep tendon reflexes.
- Pyramidal: extensor plantar responses, and distal weakness are commonly found.
- Dorsal column: Loss of vibratory and proprioceptive sensation occurs.
- Cardiac involvement occurs in 91% of patients, including cardiomegaly (up to dilated cardiomyopathy), symmetrical hypertrophy, heart murmurs, and conduction defects. Median age of death is 35 years, while females have better prognosis with a 20-year survival of 100% as compared to 63% in men.
Genetics
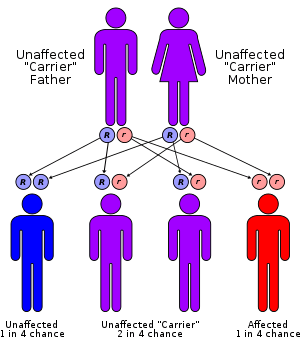
Friedreich's ataxia is an autosomal recessive disorder that occurs when the FXN gene on chromosome 9 contains amplified intronic GAA repeats (an example of Trinucleotide repeat expansion). The FXN gene encodes the protein frataxin.[4] GAA repeat expansion causes frataxin levels to be reduced and long tracts of GAA repeats induce chromosome breaks in (in vivo yeast studies). Frataxin is an iron-binding protein responsible for forming iron–sulphur clusters. One result of frataxin deficiency is mitochondrial iron overload which can cause damage to many proteins.[4] The exact role of frataxin in normal physiology remains unclear.[5]
The mutant gene contains expanded GAA triplet repeats in the first intron;[6] in a few pedigrees, point mutations have been detected. Because the defect is located in an intron (which is removed from the mRNA transcript between transcription and translation), this mutation does not result in the production of abnormal frataxin proteins. Instead, the mutation causes gene silencing (i.e., the mutation decreases the transcription of the gene) through induction of a heterochromatin structure in a manner similar to position-effect variegation.[7]
Pathology
The primary site of pathology is in the spinal cord and peripheral nerves. Sclerosis and degeneration of dorsal root ganglion, spinocerebellar tracts, lateral corticospinal tracts, and posterior columns.[8] The motor neurons of the spinal cord are spared. In peripheral nerves there is a loss of large myelinated fibres.
Progressive destruction of dorsal root ganglia accounts for thinning of dorsal roots, degeneration of dorsal columns, transsynaptic atrophy of nerve cells in Clarke's column and dorsal spinocerebellar fibers, atrophy of gracile and cuneate nuclei and neuropathy of sensory nerves. The lesion of the dentate nucleus consists of progressive and selective atrophy of large glutamatergic neurons and grumose degeneration of corticonuclear synaptic terminals that contain gamma-aminobutyric acid (GABA). Small GABA-ergic neurons and their projection fibers in the dentato-olivary tract survive. Atrophy of Betz cells and corticospinal tracts constitute a second lesion.
Pathogenesis
Low frataxin levels lead to insufficient biosynthesis of iron–sulfur clusters that are required for mitochondrial electron transport and assembly of functional aconitase and iron dysmetabolism of the entire cell. In normal individuals, the FXN gene encodes frataxin, a mitochondrial matrix protein. This globular protein consists of two α helices and seven β strands and is highly conserved, occurring in all eukaryotes and some prokaryotes.[9] Frataxin has a variety of known functions. Frataxin assists iron-sulfur protein synthesis in the electron transport chain to ultimately generate adenosine triphosphate (ATP), the energy molecule necessary to carry out metabolic functions in cells. Frataxin also regulates iron transfer in the mitochondria for providing a proper amount of reactive oxygen species (ROS) to maintain normal processes.[10] Without frataxin, the energy in the mitochondria falls, and excess iron causes extra ROS to be created, leading to further cell damage.[9][10]
DNA damage
Mitochondrial DNA (mtDNA) is especially exposed to attack by ROS since it is located within the mitochondria. Because several enzymes of the electron transport chain are encoded in mtDNA, ROS-induced damage to mtDNA may cause further increases in ROS production and oxidative stress. Elevated levels of DNA double-strand breaks have been reported in Friedreich’s ataxia patient fibroblasts and fibroblasts from a mouse model of Friedreich’s ataxia.[11] Using a lentivirus gene delivery system to deliver the frataxin gene to Friedreich’s ataxia patient and mouse model cells, it was possible to obtain long-term over-expression of frataxin mRNA and frataxin protein levels. This over-expression was associated with a substantially reduced level of DNA double-strand breaks.[11] It appears that frataxin is normally involved in the repair of DNA damage, which may be important for preventing neurodegeneration.[11]
Diagnosis
A diagnosis of Friedreich's ataxia requires investigation of the medical history and a thorough physical examination, in particular looking for balance difficulty, loss of proprioception, an absence of reflexes, and signs of other neurological problems. Genetic testing provides a conclusive diagnosis.[12] Other tests that may aid in the diagnosis or management of the disorder include:
- Electromyogram (EMG), which measures the electrical activity of muscle cells
- Nerve conduction studies, which measure the speed with which nerves transmit impulses
- Electrocardiogram (ECG), which gives a graphic presentation of the electrical activity or beat pattern of the heart
- Echocardiogram, which records the position and motion of the heart muscle
- Blood tests to check for elevated glucose levels and vitamin E levels
- Magnetic resonance imaging (MRI) or computed tomography (CT) scans, tests which provide brain and spinal cord images that are useful for ruling out other neurological conditions[12]
Treatment
Patients with Friedreich's Ataxia may require some surgical interventions (mainly for the spine and heart) with the aim to keep the patient ambulatory as long as possible. Often, titanium screws and rods are inserted in the spine to help prevent or slow the progression of scoliosis. As progression of ataxia occurs, assistive devices such as a cane, walker, or wheelchair are required for mobility and independence. Other assistive technology, such as a standing frame, can help reduce the secondary complications of prolonged use of a wheelchair.
Many patients have significant heart conditions. These conditions are much more treatable, and are often countered with ACE inhibitors such as enalapril or lisinopril and other heart medications such as digoxin.
People with Friedreich’s ataxia may benefit from a conservative treatment approach for the management of symptoms. Health professionals educated in neurological conditions, such as physical therapists and occupational therapists, can prescribe an exercise program tailored to maximize function and independence. To address the ataxic gait pattern and loss of proprioception typically seen in persons with Friedreich’s ataxia, physical therapists can use visual cueing during gait training to help facilitate a more efficient gait pattern.[13] The prescription of an assistive device along with gait training can also prolong independent ambulation.[13]
Low intensity strengthening exercises should also be incorporated to maintain functional use of the upper and lower extremities.[14] Fatigability should be monitored closely. Stabilization exercises of the trunk and low back can help with postural control and the management of scoliosis.[13] This is especially indicative if the person is non-ambulatory and requires the use of a wheelchair. Balance and coordination training using visual feedback can also be incorporated into activities of daily living.[13] Exercises should reflect functional tasks such as cooking, transfers and self-care. Along with gait training, balance and coordination training should be developed to help minimize the risk of falls.[13]
Stretching exercises can be prescribed to help relieve tight musculature due to scoliosis and pes cavus deformities.[14]
Speech therapy
Patients also often undertake speech therapy since dysarthria (a motor speech disorder) occurs in almost all Friedreich's ataxia patients. However, the dysarthria is not always ataxic and the dysarthria can be mixed. The speech intelligibility in speakers with dysarthria and Friedreich's Ataxia can be mild to severely reduced. Speech therapy seeks to improve speech outcomes and/or compensate for communication deficits.[15] Dysphagia (difficulty swallowing) is also a common symptom of Friedreich's ataxia, and speech therapy can support patients to eat and drink in a safer way.[16]
Clinical research
RG2833, a histone deacetylase inhibitor developed by Repligen, was acquired by BioMarin Pharmaceutical in January 2014.[17] A phase Ib clinical trial with RG2833 has been successfully completed in 2014 and research continues.[18]
Protection of cells from damage with the use of deuterated compounds has been attempted by Retrotope. Its first drug RT001 is a deuterated synthetic homologue of ethyl linoleate, a polyunsaturated fatty acid (11,11-D2-ethyl linoleate). Polyunsaturated fatty acids (PUFAs)are essential nutrients which are the major component of lipid membranes, particularly in mitochondria. Their high susceptibility to oxidation by reactive oxygen species through the chain reaction can be substantially reduced by the replacement of hydrogen (H) atoms with the isotope deuterium (D), yielding D-PUFAs. RT001 has been compared with non-deuterated linoleic acid ethyl ester in a randomized, double-blind, controlled trial in 18 FRDA patients for 4 weeks.[19] Primary endpoints were safety, tolerability, and pharmacokinetics. Secondary endpoints included the FARS, a timed foot-walk test and cardiopulmonary exercise testing. The study met its primary safety and tolerability endpoints.[20] An improvement in peak workload and VO2 max in the RT001 group compared to placebo, as well as a positive trend in the neurological scales in the drug group were detected, thus further development is planned.
Treatment strategies proven to be inefficient
Nicotinamide (vitamin B3) represents was found effective in preclinical FA models and well-tolerated by FA patients. An open-label, dose-escalation study demonstrated that higher doses boosted frataxin expression and attenuated abnormal heterochromatin, but failed to establish any clinical benefit in a study of 12 months. The trial is being extended.[18]
A Cochrane review on treatment of patients with Friedreich ataxia with antioxidants concluded that there is limited but not persuasive evidence of efficacy.[21] An antioxidant Idebenone was removed from the Canadian market in 2013 due to lack of effectiveness.[22]
Horizon Pharma's development plan of interferon gamma-1B for treatment of FA was given fast track designation by the Food and Drug Administration in 2015.[23] However, in the Phase 3 trial released in December 2016, the results did not meet primary endpoints. [24] The treial is to be repeated with new endpoints.
Epidemiology
Friedreich's ataxia is the most prevalent inherited ataxia,[25] affecting about 1 in 50,000 people in the United States. Males and females are affected equally. The estimated carrier prevalence is 1:110.
A 1984 Canadian study was able to trace 40 cases of classical Friedreich's disease from 14 French-Canadian kindreds previously thought to be unrelated to one common ancestral couple arriving in New France in 1634: Jean Guyon and Mathurine Robin.[26]
Epidemiological data shows that prevalence of Friedriech's ataxia follows patterns in the prevalence of haplogroup R1b. Both are more common in northern Spain, Ireland and France, rare in Russia and Scandinavia, and both follow a gradient through central and eastern Europe. This data provides an image of the prehistory of Friedreich's ataxia; a population carrying the disease went through a population bottleneck in the Franco-Cantabrian region during the last ice age. The correlation also provides a useful tool for predicting the prevalence of Friedreich's ataxia.[27]
History
Friedreich, working as a professor of pathology at the University of Heidelberg, reported five patients with the condition in a series of three papers in 1863.[28][29][30] Further observations appeared in a subsequent paper in 1876.[31]
Friedreich's ataxia was first linked to a GAA repeat expansion on chromosome 9 in 1996.[32]
Frantz Fanon wrote his medical thesis on Friedreich's ataxia, in 1951.[33]
References
- ↑ synd/1406 at Who Named It?
- ↑ Thoren C (June 1962). "Diabetes mellitus in Friedreich's ataxia". Acta Paediatrica. Supplementum. 135: 239–47. doi:10.1111/j.1651-2227.1962.tb08680.x. PMID 13921008.
- ↑ Pandolfo M (March 2009). "Friedreich ataxia: the clinical picture". Journal of Neurology. 256 Suppl 1 (1 Suppl): 3–8. doi:10.1007/s00415-009-1002-3. PMID 19283344.
- 1 2 Klockgether T (August 2011). "Update on degenerative ataxias". Current Opinion in Neurology. 24 (4): 339–45. doi:10.1097/WCO.0b013e32834875ba. PMID 21734495.
- ↑ Marmolino D (June 2011). "Friedreich's ataxia: past, present and future". Brain Research Reviews. 67 (1–2): 311–30. doi:10.1016/j.brainresrev.2011.04.001. PMID 21550666.
- ↑ Montermini L, Andermann E, Labuda M, Richter A, Pandolfo M, Cavalcanti F, Pianese L, Iodice L, Farina G, Monticelli A, Turano M, Filla A, De Michele G, Cocozza S (August 1997). "The Friedreich ataxia GAA triplet repeat: premutation and normal alleles". Human Molecular Genetics. 6 (8): 1261–6. doi:10.1093/hmg/6.8.1261. PMID 9259271.
- ↑ Friedreich Ataxia at eMedicine
- ↑ Delatycki MB, Williamson R, Forrest SM (January 2000). "Friedreich ataxia: an overview". Journal of Medical Genetics. 37 (1): 1–8. doi:10.1136/jmg.37.1.1. PMC 1734457. PMID 10633128.
- 1 2 Pandolfo M (October 2008). "Friedreich ataxia". Archives of Neurology. 65 (10): 1296–303. doi:10.1001/archneur.65.10.1296. PMID 18852343.
- 1 2 Sahdeo S, Scott BD, McMackin MZ, Jasoliya M, Brown B, Wulff H, Perlman SL, Pook MA, Cortopassi GA (December 2014). "Dyclonine rescues frataxin deficiency in animal models and buccal cells of patients with Friedreich's ataxia". Human Molecular Genetics. 23 (25): 6848–62. doi:10.1093/hmg/ddu408. PMC 4245046. PMID 25113747.
- 1 2 3 Khonsari H, Schneider M, Al-Mahdawi S, Chianea YG, Themis M, Parris C, Pook MA, Themis M (December 2016). "Lentivirus-meditated frataxin gene delivery reverses genome instability in Friedreich ataxia patient and mouse model fibroblasts". Gene Ther. 23 (12): 846–856. doi:10.1038/gt.2016.61. PMC 5143368. PMID 27518705.
- 1 2 "Friedreich's Ataxia Fact Sheet". National Institute of Neurological Disorders and Stroke.

- 1 2 3 4 5 Powers, Wendy (2007-01-01). "Holding Steady: How physical therapy can help patients with Friedreich's Ataxia". Advance. 18 (1): 26. Archived from the original on 2011-07-26. Retrieved 2011-05-16.
- 1 2 "Facts About Friedreich's Ataxia (FA)". Muscular Dystrophy Association. 2011. Archived from the original on 2011-09-27. Retrieved 2011-05-16.
- ↑ Vogel AP, Folker J, Poole ML (October 2014). "Treatment for speech disorder in Friedreich ataxia and other hereditary ataxia syndromes". The Cochrane Database of Systematic Reviews. 10 (10): CD008953. doi:10.1002/14651858.CD008953.pub2. PMID 25348587.
- ↑ Vogel AP, Brown SE, Folker JE, Corben LA, Delatycki MB (February 2014). "Dysphagia and swallowing-related quality of life in Friedreich ataxia". Journal of Neurology. 261 (2): 392–9. doi:10.1007/s00415-013-7208-4. PMID 24371004.
- ↑ "BioMarin Announces Agreement With Repligen for Pre-clinical Compounds (NASDAQ:BMRN)". Investors.bmrn.com. 2014-01-21. Archived from the original on 2015-07-05. Retrieved 2015-07-04.
- 1 2 Bürk K (2017). "Friedreich Ataxia: current status and future prospects". Cerebellum & Ataxias. 4: 4. doi:10.1186/s40673-017-0062-x. PMC 5383992. PMID 28405347.
- ↑ Indelicato E, Bosch S (2018). "Emerging therapeutics for the treatment of Friedreich's ataxia". Expert Opinion on Orphan Drugs. 6: 57. doi:10.1080/21678707.2018.1409109.
- ↑ Zesiewicz T, Heerinckx F, De Jager R, Omidvar O, Kilpatrick M, Shaw J, Shchepinov MS (April 2018). "Randomized, clinical trial of RT001: Early signals of efficacy in Friedreich's ataxia". Movement Disorders. 6 (1): 57–67. doi:10.1002/mds.27353. PMID 29624723.
- ↑ Kearney M, Orrell RW, Fahey M, Brassington R, Pandolfo M (August 2016). "Pharmacological treatments for Friedreich ataxia". The Cochrane Database of Systematic Reviews (8): CD007791. doi:10.1002/14651858.CD007791.pub4. PMID 27572719.
- ↑ "CATENA (idebenone) - Voluntary withdrawal of CATENA from the Canadian market - For the Public - Recalls & alerts - Healthy Canadians Website". Healthycanadians.gc.ca. Retrieved 2015-07-04.
- ↑ Dulaney, Chelsey (2015-04-10). "Horizon Pharma's Friedreich's Ataxia Drug Gets Fast-Track Designation". The Wall Street Journal. Retrieved 2015-07-04.
- ↑ http://www.fiercebiotech.com/biotech/horizon-slumps-after-phase-3-friedreich-s-ataxia-trial-flops%5Bfull+citation+needed%5D
- ↑ Lodi R, Tonon C, Calabrese V, Schapira AH (2006). "Friedreich's ataxia: from disease mechanisms to therapeutic interventions". Antioxidants & Redox Signaling. 8 (3–4): 438–43. doi:10.1089/ars.2006.8.438. PMID 16677089.
- ↑ Barbeau A, Sadibelouiz M, Roy M, Lemieux B, Bouchard JP, Geoffroy G (November 1984). "Origin of Friedreich's disease in Quebec". The Canadian Journal of Neurological Sciences. Le Journal Canadien Des Sciences Neurologiques. 11 (4 Suppl): 506–9. PMID 6391645.
- ↑ Vankan P (August 2013). "Prevalence gradients of Friedreich's ataxia and R1b haplotype in Europe co-localize, suggesting a common Palaeolithic origin in the Franco-Cantabrian ice age refuge". Journal of Neurochemistry. 126 Suppl 1: 11–20. doi:10.1111/jnc.12215. PMID 23859338.
- ↑ Friedreich N (1863). "Ueber degenerative Atrophie der spinalen Hinterstränge" [About degenerative atrophy of the spinal posterior column]. Arch Pathol Anat Phys Klin Med (in German). 26 (3–4): 391–419. doi:10.1007/BF01930976.
- ↑ Friedreich N (1863). "Ueber degenerative Atrophie der spinalen Hinterstränge" [About degenerative atrophy of the spinal posterior column]. Arch Pathol Anat Phys Klin Med (in German). 26 (5–6): 433–459. doi:10.1007/BF01878006.
- ↑ Friedreich N (1863). "Ueber degenerative Atrophie der spinalen Hinterstränge" [About degenerative atrophy of the spinal posterior column]. Arch Pathol Anat Phys Klin Med (in German). 27 (1–2): 1–26. doi:10.1007/BF01938516.
- ↑ Friedreich N (1876). "Ueber Ataxie mit besonderer Berücksichtigung der hereditären Formen" [About ataxia with special reference to hereditary forms]. Arch Pathol Anat Phys Klin Med (in German). 68 (2): 145–245. doi:10.1007/BF01879049.
- ↑ Campuzano V, Montermini L, Moltò MD, Pianese L, Cossée M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Cañizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M (March 1996). "Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion". Science. 271 (5254): 1423–7. doi:10.1126/science.271.5254.1423. PMID 8596916.
- ↑ Adam Shatz, "Where Life Is Seized", London Review of Books, 19 January 2017
External links
| Classification | |
|---|---|
| External resources |
- friedreichs_ataxia at NINDS
- friedreich at NIH/UW GeneTests
- NCBI Genes and Disease: Friedreich's ataxia at National Center for Biotechnology Information