Deuterated drug
A deuterated drug is a small molecule medicinal product in which one or more of the hydrogen atoms contained in the drug molecule have been replaced by its heavier stable isotope deuterium. Because of the kinetic isotope effect, deuterium-containing drugs may have significantly lower rates of metabolism, and hence a longer half-life.[1][2][3]
The first patent in the US granted for deuterated molecules was in the 1970's. Since then patents on deuterated drugs have become more prolific.[4]
History
Hydrogen is a chemical element with an atomic number of 1. It has just one proton and one electron and is the most common element. Deuterium is the heavier naturally-occurring, non-radioactive, stable isotope of hydrogen. Deuterium was discovered by Harold Urey in 1931, and he received the Nobel Prize for this discovery in 1934. The deuterium isotope effect (DIE) has become one of the most important tools that researchers use to elucidate the mechanisms of various chemical reactions. Deuterium contains one proton, one electron, and a neutron, effectively doubling the mass of the deuterium isotope without changing the steric properties significantly. However, the C-D bond is a bit shorter,[5] and it has reduced electronic polarizability and less hyperconjugative stabilization of adjacent bonds (including the developing anti-bonding orbital of a newly-forming bond). It potentially can result in weaker van der Waals stabilization, and it can produce changes that are difficult to predict, including changes in the intramolecular volume and the transition state volume.[6] Substituting deuterium for hydrogen yields deuterated compounds that are similar in size and shape to hydrogen-based compounds.
Researchers' understanding of the DIE has increased over time, and it is now applied extensively in mechanistic studies of the metabolism of drugs as well as other studies focused on pharmacokinetics (PK), efficacy, tolerability, bioavailability, and safety. The introduction of deuterated drug candidates that began in the 1970s likely evolved from earlier work with deuterated metabolites. However, it took more than 40 years for the first deuterated drug, deutetrabenazine, to be approved by the FDA.[7] Numerous publications have discussed the advantages and disadvantages of deuterated drugs.[8][9][10][11][12]
Deuterium approaches, companies, and representative compounds
Metabolic switches
Metabolic switching is a strategy to block the site of metabolism with deuterium to improve the PK and/or therapeutic profile of a drug.[13][14][15][16] Early active participants in this field included Merck, Isotechnika (now Auriniapharma.com), and BiRDs (Berlin Innovative Research and Development Services, which was renamed as Imphar AG and the acquired by Auspex). More recent studies and advances in metabolic switching have been pursued by Concert Pharmaceuticals and Auspex Pharmaceuticals (which was acquired by Teva Pharmaceuticals in 2015 for $3.5 billion).
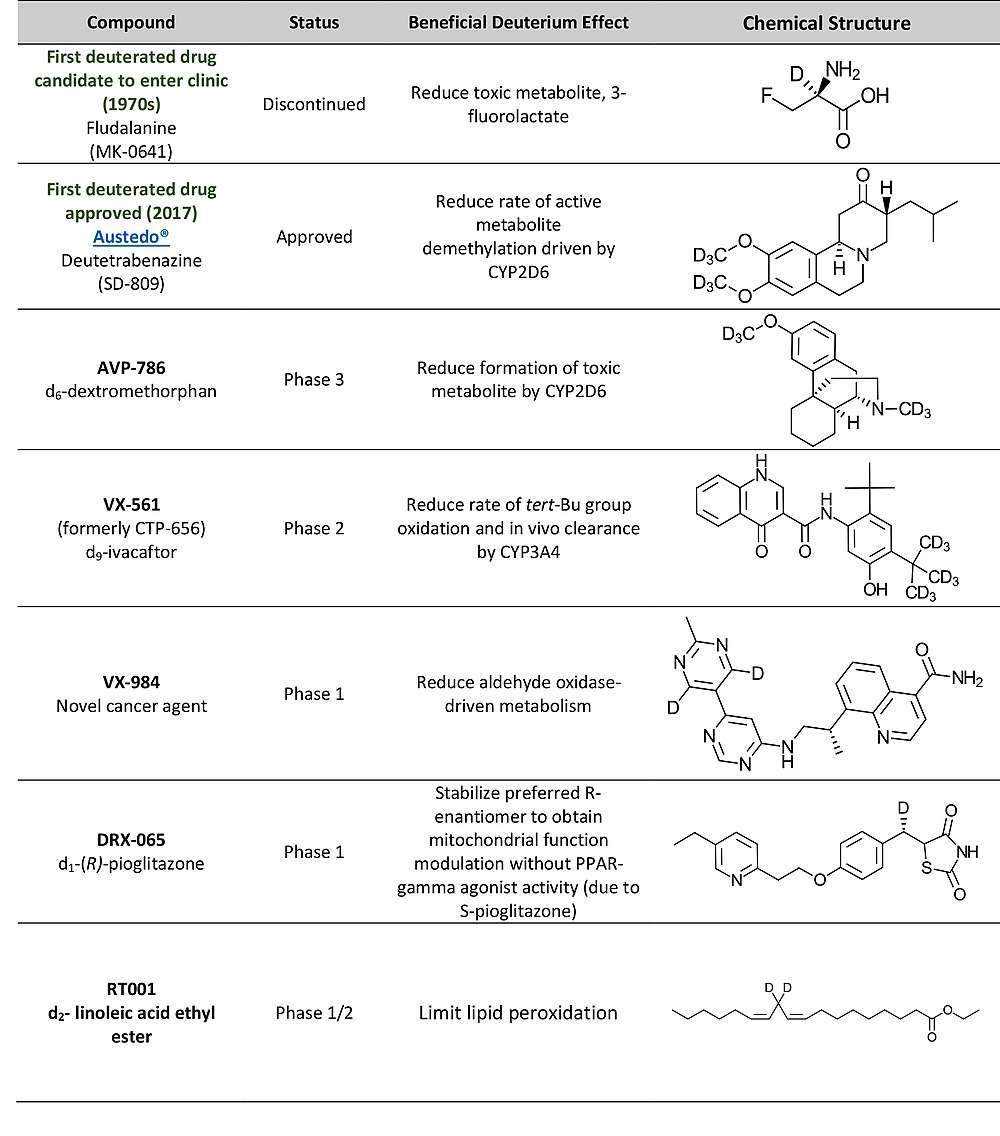
First deuterated drug candidate - Fludalanine (Merck)
In the 1970s, fludalanine was the first deuterated drug candidate to enter clinic development.[16][17][18] Janos Kollonitsch, the inventor of 3-fluoro-D-alanine, incorporated both deuterium and fluorine into alanine, something which had never been done in drug candidates before. in combination with pentizidnoe (MK-0642), fludalanine was studied as a potential antibiotic treatment in a Phase 2b clinical study in bronchitis patients with chronic obstructive pulmonary disease (COPD). In healthy subjects, deuteration significantly reduced the concentrations of the toxic metabolite, 3-fluoroactate. However, the development of fludalanine was discontinued because dosing in patients did not achieve similar reductions of 3-fluroactate.
First deuterated drug approved - Austedo®(Teva/Auspex)
Austedo®(deutetrabenazine, formerly known as SD-809), was approved in April 2017 for the treatment of chorea associated with Huntington's disease[19] and, in August 2017, it was approved for the treatment of chorea associated with tardive dyskinesia.[20] The incorporation of deuterium in tetrabenazine(Xenazine®) impedes the oxidative metabolism of the methoxy groups. Clinical studies have confirmed that the half-life of the deutetrabenazine was prolonged in vivodue to the stabilization of metabolic conversion, which resulted in slower depletion. Austedo®is dosed 2x/day, whereas tetrabenazine is dosed 3x/day. Thus, the use of Austedo®allows a lower overall dose, which reduces the risk of adverse effects.[21] Teva is also studying Austedo®in a Phase 2/3 clinical trial[22] for the potential treatment of Tourette's syndrome. Austedo was developed and approved as a New Chemical Entity(NCE) via a 505(b)(2) regulatory pathway, establishing a significant precedence for deuterated drugs (vide infra).[23][24][19][25]
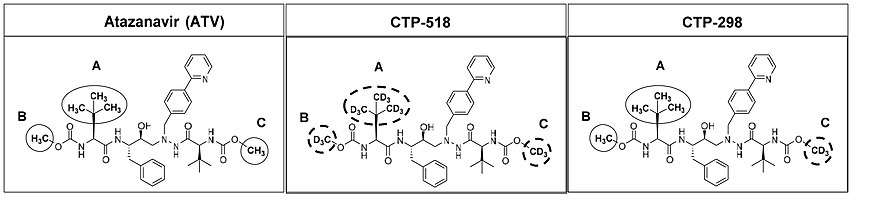
CTP-518/CTP-298 (Concert/GlaxoSmithKline(GSK))
Concert synthesized and tested several deuterated analogs of atazanavir to block sites of metabolism. in preclinical studies in human liver microsome ("HLM" studies), the half-life of CTP-518 compared to atazanavir was extended by 60%, while the half-life of CTP-298 was extended by only 12%. However, in human PK crossover studies, CTP-298 was more stable metabolically than either CTP-518 or atazanavir, which CTP-518 was considerably worse than atazanavir in terms of plasma exposure. These results demonstrated that the effect of deuterium substitution at a drug's known "metabolism hotspots" is unpredictable.[26]
CTP-543 (Concert)
Deuterated ruxolitinib is being studied by Concert for the potential treatment of alopecia areata in a Phase 2 clinical trial.[28] In January 2018, the FDA granted Fast Track designation to CTP-543. The parent non-deuterated drug, ruxolitinib (Jakafi®), was developed by Incyte, and it is approved for the treatment of high-risk myelofibrosis. The chemical structure of CTP-543 has not been disclosed. In human clinical trials, CTP-543 demonstrated statistically significant improvement in several PK measures, including half-life, clearance, and area under the curve(AUC) for CTP-543 compared to ruxolitinib.[29]
Both Concert and Incyte have been issued U.S. patents for deuterated analogs of ruxolitinib. In April 2017, Incyte filed an Inter Partes Review Petition (IPR) against Concert, seeking to invalidate Concert's U.S. Patent No. 9,249,149. In October 2017, the U.S. Patent and Trademark Review Board (PTAB) denied Incyte's petition to institute IPR. In April 2018, the PTAB granted Incyte's motion for reconsideration. in June 2017, Concert filed a Post Grant Review Petition (PGR) against Incyte Corporation seeking to invalidate Incyte's U.S. Patent No. 9,662,335. In January 2017, the PTAB made the decision not to institute the PGR proceeding.[30]
BMS-986165 (Bristol Myers Squibb)
BMS-986165 is a novel deuterated compound that is an oral allosteric inhibitor of TYK2 and is currently in Phase 2 clinical trials for the treatment of Systemic Lupus Erythematosus.[31] The chemical structure has not been disclosed.
AVP-786 (Concert/Avanir)
AVP-786 is a deuterated analog of dextromethorphan that is being studied for the potential treatment of agitation in Alzheimer's disease patients. A metabolite of dextromethorphan, dextrorphan, is responsible for the drug's clinical activity, but it has toxic properties. In addition, researchers have found that unmetabolized dextromethorphan offers promise as an NMDA receptor antagonist. Concert and Avanir Pharmaceuticals are collaborating on a combination of AVP-786 with quinine, Nuedexta, which has demonstrated slower formation of the toxic metabolite while enhancing exposure to dextromethorphan.
VX-561 (Vertex/Concert)
Vx-561 is a deuterated analog of ivacaftor that is being studied for the potential treatment of patients with cystic fibrosis. Ivacaftor (Kalydeco®) was the first drug approved for the treatment of Cystic Fibrosis(CF) in patients with specific mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In people, deuteration of ivcaftor's tert-butyl groups to create CTP-656 leads to greater than a 3-fold increase in exposure to ivacaftor, a marked increase in half-life, and greater plasma levels at 24 hours.[32]Vertex acquired VX-561 (formerly CTP-656) from Concert in 2017 for $260 million.
SD-1077 (Teva/Auspex/BiRDs)
SD-1077 is a deuterated analog of L-dopa for the potential treatment of Parkinson's disease and movement disorders. Deuterium substitutions in the L-DOPA molecule yield dopamine that appeared to be more resistant to enzymatic breakdown.[33] Teva obtained SD-1077 through the acquisition of Auspex, which acquired the compound from Imphar AG/BiRDs in 2015.
JZP-386 (Concert/Jazz)
JZP-386 is a deuterated analog of sodium oxybate for the potential treatment of narcolepsy. Concert signed a collaboration agreement with Jazz Pharmaceuticals in 2013, and in 2015 they were "exploring formulation options designed to leverage the positive effects observed with JXP-386 in Phase 1 trials."
VX-984 (Merck KGa/Vertex)
VX-984 is a novel, DNA-dependent protein kinase inhibitor originally discovered by Vertex, which was acquired by Merck KGa in 2017. A Phase 1 study was completed in 2017.[34]
Chiral Switching
The approach of advancing the preferred, stable stereoisomer out of a racemic mixture is known as "chiral switching," and it was the business model for Sepracor (now Sunovion). Sepracor built a pharmaceutical business that generated more than $1 billion in annual revenue based on three "chiral switches,", i.e., Lunesta®(eszopiclone), Xopenex®(levabuterol hydrochloride), and Brovana® argormoterol tartrate). Sepracor was acquired by Sunovion Pharmaceuticals in 2010. Several other successful "chiral switches" include Nexium®from Prilosec® (AstraZeneca), Lexapro®from Celexa®(Forest Labs), and Fetzima®from Savella®(Forest Labs).
Some stereoisomers chemically interconvert (in vitro and in vivo) and therefore, chiral switching is impossible. Representative racemic compounds include thalidomide compounds (linalidomide, pomalidomide, avadomide), thiazolidinedione analogs (rosiglitazone, pioglitazone, MSDC-0602, lobeglitazone), bupropion, donepezil, and prasugrel. Stabilization at a chiral center with deuterium may enable characterization of stereoisomers prone to interconversion (designed to slow racemization). This approach of "deuterium-enabled chiral switching" (DECS) was pioneered by Deuteria Pharmaceuticals (acquired by Celgene in 2012) continued by DeuteRx.
DRX-065 (DeuteRx)
The deuterium-stabilized R-enantiomer of pioglitazone, DRX-065, currently is in Phase 1 at DeuteRX, and it is being pursued for adrenomveloneuropathy (AMN) and nonalcoholic steatohepatitis (NASH). The two enantiomers of pioglitazone were found to have quite different clinical characteristics that could not have been predicted a priori. Both enantiomers are mitochondria function modulators. However, R-pioglitazone is responsible exclusively for the NASH efficacy, while S-pioglitazone is responsible for the PPARy activity and associated with side effects (weight gain, edema).[35]
DRX-164 (DeuteRx)
The deuterium-stabilized S-enantiomer of avadomide, DRX-164, is in preclinical development for hematological malignancies and solid tumors at DeuteRx. The parent drug, avadomide (CC-122),[36] is a racemic cereblon E3 ligase modulator (CELMoD®) that currently is in development at Celgene for hematological malignancies and solid tumors. DeuteRx published preclinical results demonstrating that the anti-inflammatory and anti-tumorigenic effects of avadomide were due exclusively to the S-enantiomer.[37] Similar effects have been observed for the deuterium-stabilized enantiomers of lenalidomide (Revlimid) in an earlier program at Deuteria Pharmaceuticals.[38]
Inhibition of Chemical Processes
ALK001 (Alkeus)
Incorporation of deuterium at the C20 position of vitamin A to create ALK001 slows the formation of detrimental vitamin A dimers.[39] ALK001 is now in Phase 2 clinical trials for Stargardt disease, a major cause of juvenile blindness. It will be tested in a Phase 2/3 trial for age-related macular degeneration(AMD) by 2019. Interestingly, Josha Boger, the chairman of Alkeus Pharmaceuticals, is the founder and former CEO of Vertex, and he previously worked at Merck, as have several other contributors to deuterated drug candidates.
RT001 (Retrotope)
Retrotope is pioneering the use of deuterated fatty acids to limit lipid peroxidation which protects against free radical damage and cell death, a hallmark of several degenerative diseases. Fatty acids in cell membranes are a source of reactive oxygen species, and deuterated analogs may be less prone to generating them.[40][41] RT001 is a di-deuterated linoleum acid ethyl ester and has demonstrated early efficacy in a Phase 1/2 clinical trial for the treatment of Friedreich's ataxia.[42]
Intellectual Property
Isotechnika, BiRDs, Auspex, Concert, Deuteria Pharmaceuticals, and DeuteRx have navigated completely new patent territory. From 2007 through 2009, more than 400 patent applications related to deuterated drug candidates were filed by Auspex, Concert, and Protia (original IP holding company for Deuteria Pharmaceuticals). In 2009, Dr. Czarnik alone filed 220 patent applications for deuterated drugs, which made him one of the most prolific U.S. inventors that year.
Case law related to the validity of deuterated drug patents is continuing to evolve. Since 2015, there have been three challenges filed with the U.S. Patent and Trademark Board (PTAB) related to deuterated drug patents. In 2015, the PTAB denied Neptune Generics Inter Parties' Review Petition (IPR2015-0313) in which the company sought to invalidate Auspex's U.S. Patent No. 7,456,317 with claims covering deuterated analogs of venlafaxine. In 2017, the PTAB denied Incyte's request for reconsideration. In 2018, the PTAB denied Concert's Post Grant Review Petition (PGR2017-00034R) against Incyte in which it sought to invalidate Incyte's U.S. Patent No. 9,662,335 with claims covering deuterated analogs of ruxolitinib.[43]
Regulatory Strategy
Based on precedent with the Austedo®approval, it is likely that deuterated drugs will be considered as NCEs by the FDA.
Also, if the non-deuterated drug is already on the market, the deuterated drug candidate may be able to pursue a 505(b)(2) regulatory pathway at the FDA. Investigators can follow the 505(b)(2) pathway for approval of a deuterated drug based on the approved non-deuterated drug. This regulatory pathway allows referencing some non-clinical and safety findings reported in the New Drug Application(NDA) for the parent non-deuterated drug. Additional precedence for the 505(b)(2) regulatory pathway has been demonstrated with "chiral switches," including Xyzal®, Xopenex®, and Lexapro®. the 505(b)(2) or similar strategy has been or is being pursued for several deuterated drug candidates, including Austedo®, AVP-786, RT001, CTP-656, CTP-543, and DRX-065.
Further reading
References
- ↑ Sanderson K (March 2009). "Big interest in heavy drugs". Nature. 458 (7236): 269. doi:10.1038/458269a. PMID 19295573.
- ↑ Katsnelson A (June 2013). "Heavy drugs draw heavy interest from pharma backers". Nature Medicine. 19 (6): 656. doi:10.1038/nm0613-656. PMID 23744136.
- ↑ Gant TG (May 2014). "Using deuterium in drug discovery: leaving the label in the drug". Journal of Medicinal Chemistry. 57 (9): 3595–611. doi:10.1021/jm4007998. PMID 24294889.
- ↑ "Drugs that live long will prosper". The Economist. ISSN 0013-0613. Retrieved 2015-09-18.
- ↑ "Electron‐Diffraction Study of the Structures of C2H4 and C2D4". The Journal of Chemical Physics.
- ↑ "Using Deuterium in Drug Discovery: Leaving the Label in the Drug". The Journal of Medicinal Chemistry.
- ↑ "A Decade of Deuteration in Medicinal Chemistry". Annual Reports in Medicinal Chemistry.
- ↑ Liu, JF; Harbeson, SL; Brummel, CL; Tung, R; Doller, D (2017). "A Decade of Deuteration in Medicinal Chemistry". Annual Reports in Medicinal Chemistry. 50.
- ↑ Foster, AB (1985). "Deuterium Isotope Effects". Adv. Drug. Res. 14.
- ↑ Sanderson, K. "Big interest in heavy drugs". Nature. 458.
|access-date=requires|url=(help) - ↑ Katsnelson, A. "Heavy drugs draw heavy interest from pharma backers". Nature Medicine. 19.
|access-date=requires|url=(help) - ↑ Gant, TG. "Using deuterium in drug discovery: leaving the label in the drug". Journal of Medicinal Chemistry. 57.
|access-date=requires|url=(help) - ↑ Foster, Allan B (1984-01-01). "Deuterium isotope effects in studies of drug metabolism". Trends in Pharmacological Sciences. 5: 524–527. doi:10.1016/0165-6147(84)90534-0. ISSN 0165-6147.
- ↑ B., Foster A. (1986-08-10). "Deuterium isotope effects in the metabolism of drugs and xenobiotics implications for drug design". Testa, B (Ed ) Advances in Drug Research, Vol 14 Ix+339p Academic Press, Inc.
- ↑ Morgan, Adam J.; Nguyen, Sophia; Uttamsingh, Vinita; Bridson, Gary; Harbeson, Scott; Tung, Roger; Masse, Craig E. (July 2011). "Design and synthesis of deuterated boceprevir analogs with enhanced pharmacokinetic properties". Journal of Labelled Compounds and Radiopharmaceuticals. 54 (9): 613–624. doi:10.1002/jlcr.1905. ISSN 0362-4803.
- 1 2 Darland, G. K.; Hajdu, R.; Kropp, H.; Kahan, F. M.; Walker, R. W.; Vandenheuvel, W. J. (1986-11-01). "Oxidative and defluorinative metabolism of fludalanine, 2-2H-3-fluoro-D-alanine". Drug Metabolism and Disposition. 14 (6): 668–673. ISSN 0090-9556. PMID 2877824.
- ↑ "Hallelujah Moments | Eugene H. Cordes | 9780199337149 | Oxford University Press Canada". www.oupcanada.com. Retrieved 2018-09-11.
- ↑ "A Deuterated Drug That Almost Succeeded | Chemical & Engineering News". cen.acs.org. Retrieved 2018-09-11.
- 1 2 "Drugs@FDA: FDA Approved Drug Products". www.accessdata.fda.gov. Retrieved 2018-09-11.
- ↑ "Drugs@FDA: FDA Approved Drug Products". www.accessdata.fda.gov. Retrieved 2018-09-11.
- ↑ Claassen, Daniel O.; Carroll, Benjamin; De Boer, Lisa M.; Wu, Eric; Ayyagari, Rajeev; Gandhi, Sanjay; Stamler, David (2017-03-01). "Indirect tolerability comparison of Deutetrabenazine and Tetrabenazine for Huntington disease". Journal of Clinical Movement Disorders. 4 (1): 3. doi:10.1186/s40734-017-0051-5. ISSN 2054-7072. PMC 5331691. PMID 28265459.
- ↑ "Alternatives for Reducing Tics in TS: A Study of TEV-50717 (Deutetrabenazine) for the Treatment of Tourette Syndrome in Children and Adolescents. - Full Text View - ClinicalTrials.gov". Retrieved 2018-09-11.
- ↑ Schmidt, Charles (7 June 2017). "First deuterated drug approved". Nature Biotechnology. 35 (6): 493–494. doi:10.1038/nbt0617-493. ISSN 1546-1696. PMID 28591114.
- ↑ LLP, Pepper Hamilton. "Deuterated Drugs Are New Chemical Entities". Pepper Hamilton LLP. Retrieved 2018-09-11.
- ↑ DeWitt, Sheila H.; Maryanoff, Bruce E. (2017-11-21). "Deuterated Drug Molecules: Focus on FDA-Approved Deutetrabenazine". Biochemistry. 57 (5): 472–473. doi:10.1021/acs.biochem.7b00765. ISSN 0006-2960. PMID 29160059.
- ↑ "CONCERT PHARMACEUTICALS, INC.'S PATENT OWNER PRELIMINARY RESPONSE" (PDF).
- ↑ "S-1". www.sec.gov. Retrieved 2018-09-11.
- ↑ "Study to Evaluate the Safety and Efficacy of CTP-543 in Adult Patients With Moderate to Severe Alopecia Areata - Full Text View - ClinicalTrials.gov". Retrieved 2018-09-11.
- ↑ "CONCERT PHARMACEUTICALS, INC.'S PATENT OWNER PRELIMINARY RESPONSE" (PDF).
- ↑ "Intellectual Property - Concert Pharmaceuticals". Concert Pharmaceuticals. Retrieved 2018-09-11.
- ↑ "An Investigational Study to Evaluate BMS-986165 in Patients With Systemic Lupus Erythematosus - Full Text View - ClinicalTrials.gov". Retrieved 2018-09-11.
- ↑ Harbeson, Scott L.; Morgan, Adam J.; Liu, Julie F.; Aslanian, Ara M.; Nguyen, Sophia; Bridson, Gary W.; Brummel, Christopher L.; Wu, Lijun; Tung, Roger D. (2017-08-01). "Altering Metabolic Profiles of Drugs by Precision Deuteration 2: Discovery of a Deuterated Analog of Ivacaftor with Differentiated Pharmacokinetics for Clinical Development". Journal of Pharmacology and Experimental Therapeutics. 362 (2): 359–367. doi:10.1124/jpet.117.241497. ISSN 0022-3565. PMID 28611092.
- ↑ Malmlöf, Torun; Feltmann, Kristin; Konradsson-Geuken, Åsa; Schneider, Frank; Alken, Rudolf-Giesbert; Svensson, Torgny H.; Schilström, Björn (2014-06-07). "Deuterium-substituted l-DOPA displays increased behavioral potency and dopamine output in an animal model of Parkinson's disease: comparison with the effects produced by l-DOPA and an MAO-B inhibitor". Journal of Neural Transmission. 122 (2): 259–272. doi:10.1007/s00702-014-1247-6. ISSN 0300-9564. PMID 24906468.
- ↑ "First-in-Human Study of the Safety, Tolerability, and Pharmacokinetic/Pharmacodynamic Profile of VX-984 in Combination With Chemotherapy - Full Text View - ClinicalTrials.gov". Retrieved 2018-09-11.
- ↑ "Revolutionary Approach | DeuteRx". deuterx.com. Retrieved 2018-09-11.
- ↑ Kritharis, Athena; Coyle, Michael; Sharma, Jaya; Evens, Andrew M. (2015-04-16). "Lenalidomide in non-Hodgkin lymphoma: biological perspectives and therapeutic opportunities". Blood. 125 (16): 2471–2476. doi:10.1182/blood-2014-11-567792. ISSN 0006-4971. PMC 4467884. PMID 25736312.
- ↑ Jacques, Vincent; Czarnik, Anthony W.; Judge, Thomas M.; Van der Ploeg, Lex H. T.; DeWitt, Sheila H. (2015-03-24). "Differentiation of antiinflammatory and antitumorigenic properties of stabilized enantiomers of thalidomide analogs". Proceedings of the National Academy of Sciences of the United States of America. 112 (12): E1471–1479. doi:10.1073/pnas.1417832112. ISSN 1091-6490. PMC 4378388. PMID 25775521.
- ↑ "Understanding the Class of Thalidomide Analogs Through the Stabilization & Characterization of Their Interconverting Enantiomers" (PDF).
- ↑ Kaufman, Yardana; Ma, Li; Washington, Ilyas (2011-03-11). "Deuterium Enrichment of Vitamin A at the C20 Position Slows the Formation of Detrimental Vitamin A Dimers in Wild-type Rodents". Journal of Biological Chemistry. 286 (10): 7958–7965. doi:10.1074/jbc.M110.178640. ISSN 0021-9258. PMC 3048682. PMID 21075840.
- ↑ "Pill of super-protective 'heavy' fat may be key to eternal youth". New Scientist. Retrieved 2018-09-12.
- ↑ "RT 001 - AdisInsight". adisinsight.springer.com. Retrieved 2018-09-12.
- ↑ Zesiewicz, Theresa; Heerinckx, Frederic; De Jager, Robert; Omidvar, Omid; Kilpatrick, Marcus; Shaw, Jessica; Shchepinov, Mikhail S. (2018-04-06). "Randomized, clinical trial of RT001: Early signals of efficacy in Friedreich's ataxia". Movement Disorders. 33 (6): 1000–1005. doi:10.1002/mds.27353. ISSN 0885-3185. PMID 29624723.
- ↑ "Intellectual Property - Concert Pharmaceuticals". Concert Pharmaceuticals. Retrieved 2018-09-12.