CD28
CD28 (Cluster of Differentiation 28) is one of the proteins expressed on T cells that provide co-stimulatory signals required for T cell activation and survival. T cell stimulation through CD28 in addition to the T-cell receptor (TCR) can provide a potent signal for the production of various interleukins (IL-6 in particular).
CD28 is the receptor for CD80 (B7.1) and CD86 (B7.2) proteins. When activated by Toll-like receptor ligands, the CD80 expression is upregulated in antigen-presenting cells (APCs). The CD86 expression on antigen-presenting cells is constitutive (expression is independent of environmental factors).
CD28 is the only B7 receptor constitutively expressed on naive T cells. Association of the TCR of a naive T cell with MHC:antigen complex without CD28:B7 interaction results in a T cell that is anergic.
Signaling
CD28 possesses an intracellular domain with several residues that are critical for its effective signaling. The YMNM motif beginning at tyrosine 170 in particular is critical for the recruitment of SH2-domain containing proteins, especially PI3K,[4] Grb2[5] and Gads. The Y170 residue is important for the induction of Bcl-xL via mTOR and enhancement of IL-2 transcription via PKCθ, but has no effect on proliferation and results a slight reduction in IL-2 production. The N172 residue (as part of the YMNM) is important for the binding of Grb2 and Gads and seems to be able to induce IL-2 mRNA stability but not NF-κB translocation. The induction of NF-κB seems to be much more dependent on the binding of Gads to both the YMNM and the two proline-rich motifs within the molecule. However, mutation of the final amino acid of the motif, M173, which is unable to bind PI3K but is able to bind Grb2 and Gads, gives little NF-κB or IL-2, suggesting that those Grb2 and Gads are unable to compensate for the loss of PI3K. IL-2 transcription appears to have two stages; a Y170-dependent, PI3K-dependent initial phase which allows transcription and a PI3K-independent second phase which is dependent on formation of an immune synapse, which results in enhancement of IL-2 mRNA stability. Both are required for full production of IL-2.
CD28 also contains two proline-rich motifs that are able to bind SH3-containing proteins. Itk and Tec are able to bind to the N-terminal of these two motifs which immediately succeeds the Y170 YMNM; Lck binds the C-terminal. Both Itk and Lck are able to phosphorylate the tyrosine residues which then allow binding of SH2 containing proteins to CD28. Binding of Tec to CD28 enhances IL-2 production, dependent on binding of its SH3 and PH domains to CD28 and PIP3 respectively. The C-terminal proline-rich motif in CD28 is important for bringing Lck and lipid rafts into the immune synapse via filamin-A. Mutation of the two prolines within the C-terminal motif results in reduced proliferation and IL-2 production but normal induction of Bcl-xL. Phosphorylation of a tyrosine within the PYAP motif (Y191 in the mature human CD28) forms a high affinity-binding site for the SH2 domain of the src kinase Lck which in turn binds to the serine kinase PKC-θ.[6]
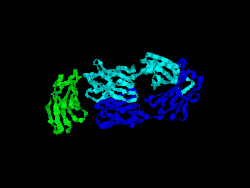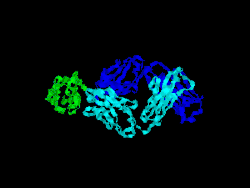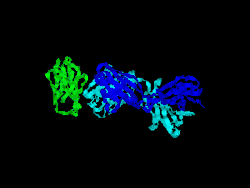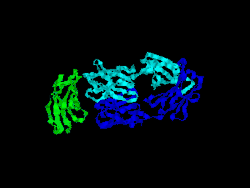
Structure
The first structure of CD28 was obtained in 2005 by the T-cell biology group at the University of Oxford.[7]
As a drug target
The drug TGN1412, which was produced by the German biotech company TeGenero, and unexpectedly caused multiple organ failure in trials, is a superagonist of CD28. Unfortunately, it is often ignored that the same receptors also exist on cells other than lymphocytes. CD28 has also been found to stimulate eosinophil granulocytes where its ligation with anti-CD28 leads to the release of IL-2, IL4, IL-13 and IFN-γ.[8][9]
References
- GRCh38: Ensembl release 89: ENSG00000178562 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Prasad KV, Cai YC, Raab M, Duckworth B, Cantley L, Shoelson SE, Rudd CE (Mar 1994). "T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif". Proceedings of the National Academy of Sciences of the United States of America. 91 (7): 2834–8. doi:10.1073/pnas.91.7.2834. PMC 43465. PMID 8146197.
- Schneider H, Cai YC, Prasad KV, Shoelson SE, Rudd CE (Apr 1995). "T cell antigen CD28 binds to the GRB-2/SOS complex, regulators of p21ras". European Journal of Immunology. 25 (4): 1044–50. doi:10.1002/eji.1830250428. PMID 7737275.
- Kong KF, Yokosuka T, Canonigo-Balancio AJ, Isakov N, Saito T, Altman A (Nov 2011). "A motif in the V3 domain of the kinase PKC-θ determines its localization in the immunological synapse and functions in T cells via association with CD28". Nature Immunology. 12 (11): 1105–12. doi:10.1038/ni.2120. PMC 3197934. PMID 21964608.
- Evans EJ, Esnouf RM, Manso-Sancho R, Gilbert RJ, James JR, Yu C, Fennelly JA, Vowles C, Hanke T, Walse B, Hünig T, Sørensen P, Stuart DI, Davis SJ (March 2005). "Crystal structure of a soluble CD28-Fab complex". Nat. Immunol. 6 (3): 271–9. doi:10.1038/ni1170. PMID 15696168.
- Woerly G, Roger N, Loiseau S, Dombrowicz D, Capron A, Capron M (1999). "Expression of Cd28 and Cd86 by Human Eosinophils and Role in the Secretion of Type 1 Cytokines (Interleukin 2 and Interferon γ): Inhibition by Immunoglobulin a Complexes". J Exp Med. 190 (4): 487–95. doi:10.1084/jem.190.4.487. PMC 2195599. PMID 10449520.
- Woerly G, Lacy P, Younes AB, Roger N, Loiseau S, Moqbel R, Capron M (2002). "Human eosinophils express and release IL-13 following CD28-dependent activation". J Leukoc Biol. 72 (4): 769–79. doi:10.1189/jlb.72.4.769. PMID 12377947.
- Ellis JH, Ashman C, Burden MN, Kilpatrick KE, Morse MA, Hamblin PA (June 2000). "GRID: a novel Grb-2-related adapter protein that interacts with the activated T cell costimulatory receptor CD28". J. Immunol. 164 (11): 5805–14. doi:10.4049/jimmunol.164.11.5805. PMID 10820259.
- Okkenhaug K, Rottapel R (August 1998). "Grb2 forms an inducible protein complex with CD28 through a Src homology 3 domain-proline interaction". J. Biol. Chem. 273 (33): 21194–202. doi:10.1074/jbc.273.33.21194. PMID 9694876.
- Nunès JA, Truneh A, Olive D, Cantrell DA (January 1996). "Signal transduction by CD28 costimulatory receptor on T cells. B7-1 and B7-2 regulation of tyrosine kinase adaptor molecules". J. Biol. Chem. 271 (3): 1591–8. doi:10.1074/jbc.271.3.1591. PMID 8576157.
- Pagès F, Ragueneau M, Klasen S, Battifora M, Couez D, Sweet R, Truneh A, Ward SG, Olive D (April 1996). "Two distinct intracytoplasmic regions of the T-cell adhesion molecule CD28 participate in phosphatidylinositol 3-kinase association". J. Biol. Chem. 271 (16): 9403–9. doi:10.1074/jbc.271.16.9403. PMID 8621607.
Further reading
- Linsley PS, Ledbetter JA (1993). "The role of the CD28 receptor during T cell responses to antigen". Annu. Rev. Immunol. 11: 191–212. doi:10.1146/annurev.iy.11.040193.001203. PMID 8386518.
- Lenschow DJ, Walunas TL, Bluestone JA (1996). "CD28/B7 system of T cell costimulation". Annu. Rev. Immunol. 14: 233–58. doi:10.1146/annurev.immunol.14.1.233. PMID 8717514.
- Greenfield EA, Nguyen KA, Kuchroo VK (1998). "CD28/B7 costimulation: a review". Crit. Rev. Immunol. 18 (5): 389–418. doi:10.1615/critrevimmunol.v18.i5.10. PMID 9784967.
- Chang TT, Kuchroo VK, Sharpe AH (2002). Role of the B7-CD28/CTLA-4 pathway in autoimmune disease. Curr. Dir. Autoimmun. Current Directions in Autoimmunity. 5. pp. 113–30. doi:10.1159/000060550. ISBN 978-3-8055-7308-5. PMID 11826754.
- Bour-Jordan H, Blueston JA (2002). "CD28 function: a balance of costimulatory and regulatory signals". J. Clin. Immunol. 22 (1): 1–7. doi:10.1023/A:1014256417651. PMID 11958588.
- Greenway AL, Holloway G, McPhee DA, Ellis P, Cornall A, Lidman M (2004). "HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication". J. Biosci. 28 (3): 323–35. doi:10.1007/BF02970151. PMID 12734410.
- Bénichou S, Benmerah A (2003). "[The HIV nef and the Kaposi-sarcoma-associated virus K3/K5 proteins: "parasites"of the endocytosis pathway]". Med Sci (Paris). 19 (1): 100–6. doi:10.1051/medsci/2003191100. PMID 12836198.
- Tolstrup M, Ostergaard L, Laursen AL, Pedersen SF, Duch M (2004). "HIV/SIV escape from immune surveillance: focus on Nef". Curr. HIV Res. 2 (2): 141–51. doi:10.2174/1570162043484924. PMID 15078178.
- Anderson JL, Hope TJ (2005). "HIV accessory proteins and surviving the host cell". Current HIV/AIDS Reports. 1 (1): 47–53. doi:10.1007/s11904-004-0007-x. PMID 16091223.
- Li L, Li HS, Pauza CD, Bukrinsky M, Zhao RY (2006). "Roles of HIV-1 auxiliary proteins in viral pathogenesis and host-pathogen interactions". Cell Res. 15 (11–12): 923–34. doi:10.1038/sj.cr.7290370. PMID 16354571.
- Stove V, Verhasselt B (2006). "Modelling thymic HIV-1 Nef effects". Curr. HIV Res. 4 (1): 57–64. doi:10.2174/157016206775197583. PMID 16454711.
External links
- Mouse CD Antigen Chart
- Human CD Antigen Chart
- Human CD28 genome location and CD28 gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: P10747 (T-cell-specific surface glycoprotein CD28) at the PDBe-KB.