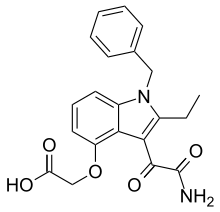
Varespladib
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H20N2O5 |
| Molar mass | 380.4 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Varespladib is an inhibitor of the IIa, V, and X isoforms of secretory phospholipase A2 (sPLA2).[1][2][3] The molecule acts as an anti-inflammatory agent by disrupting the first step of the arachidonic acid pathway of inflammation.[4] From 2006 to 2012, varespladib was under active investigation by Anthera Pharmaceuticals as a potential therapy for several inflammatory diseases, including acute coronary syndrome and acute chest syndrome.[5][6] The trial was halted in March 2012 due to inadequate efficacy.[7]
Oral varespladib
Intravenous varespladib
 | |
| Clinical data | |
|---|---|
| Synonyms | A-001 |
| Pregnancy category |
|
| Routes of administration | IV |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H19N2NaO5 |
| Molar mass | 402.4 g/mol |
| 3D model (JSmol) | |
| |
| |
Varespladib sodium (also known as A-001, previously LY315920 and S-5920) is a sodium salt of varespladib designed for intravenous delivery.[8] It was under evaluation by Anthera Pharmaceuticals as an anti-inflammatory sPLA2 inhibitor for the prevention of acute chest syndrome (ACS), the leading cause of death for patients with sickle-cell disease.[5]
Elevated serum levels of sPLA2 have been observed in sickle-cell patients preceding and during ACS episodes. This profound elevation in sPLA2 levels is not observed in sickle-cell patients at steady-state or during a vaso-occlusive crisis, or in patients with respiratory diseases such as pneumonia.[9][10] A reduction in serum sPLA2 levels, for example through blood transfusion, reduces the risk of an ACS, suggesting that sPLA2 plays an important role in the onset of ACS.[11]
Anthera Pharmaceuticals acquired varespladib sodium from Lilly and Shionogi in 2006.[5] In 2007, the U.S. Food and Drug Administration (FDA) granted varespladib sodium orphan drug status for its potential to treat patients with sickle-cell disease.[12] In 2009, Anthera Pharmaceuticals completed a Phase II study of varespladib sodium in subjects with sickle cell disease at risk for ACS.[13]
References
- ↑ "Following Encouraging Results, Anthera to Continue IMACTS Trial for the Prevention of Acute Chest Syndrome in Patients with Sickle Cell Disease" (Press release). Anthera Pharmaceuticals, Inc. 24 March 2009.
- ↑ "A-002: Short Term (16 week) Treatment of Acute Coronary Syndrome". Anthera Pharmaceuticals. Retrieved 6 September 2011.
- ↑ "Varespladib". American Journal of Cardiovascular Drugs. 11 (2): 137–43. April 2011. doi:10.2165/11533650-000000000-00000. PMID 21446779.
- ↑ Baynes JW, Dominiczak MH (2005). Medical Biochemistry (2 ed.). Elsevier Mosby. p. 555. ISBN 0-7234-3341-0.
- 1 2 3 "Anthera Licenses Portfolio of Anti-Inflammatory Products From Eli Lilly and Company and Shionogi & Co., Ltd" (Press release). Anthera Pharmaceuticals, Inc. 6 September 2006.
- ↑ "Science: sPLA2". Anthera Pharmaceuticals. Retrieved 6 August 2011.
- ↑ ClinicalTrials.gov. "VISTA-16 Trial: Evaluation of Safety and Efficacy of Short-term A-002 Treatment in Subjects With Acute Coronary Syndrome". United States National Institute of Health. Retrieved 2011-08-17.
- ↑ "A-001: Prevention of Acute Chest Syndrome in Sickle Cell Disease". Anthera Pharmaceuticals. Retrieved 18 August 2011.
- ↑ Styles LA, Schalkwijk CG, Aarsman AJ, Vichinsky EP, Lubin BH, Kuypers FA (March 1996). "Phospholipase A2 levels in acute chest syndrome of sickle cell disease" (PDF). Blood. 87 (6): 2573–78. PMID 8630425.
- ↑ Styles LA, Aarsman AJ, Vichinsky EP, Kuypers FA (November 2000). "Secretory phospholipase A(2) predicts impending acute chest syndrome in sickle cell disease". Blood. 96 (9): 3276–78. PMID 11050014.
- ↑ Bostrom M, Boyanovsky B, Jordan C, Wadsworth M, Taatjes D, de Beer F, et al. (March 2007). "Group v secretory phospholipase A2 promotes atherosclerosis: evidence from genetically altered mice". Arteriosclerosis, Thrombosis, and Vascular Biology. 27 (3): 600–06. doi:10.1161/01.ATV.0000257133.60884.44. PMID 17204667.
- ↑ "Anthera's A-001 Receives Orphan Drug Status For The Prevention Of Acute Chest Syndrome In Patients With Sickle Cell Disease" (Press release). Anthera Pharmaceuticals, Inc. 18 December 2007.
- ↑ ClinicalTrials.gov. "IMPACTS Trial: Investigation of the Modulation of Phospholipase in Acute Chest Syndrome". United States National Institute of Health. Retrieved 18 August 2011.