Uroporphyrinogen III
 | |
| Identifiers | |
|---|---|
| MeSH | Uroporphyrinogen+III |
PubChem CID |
|
| Properties | |
| C40H44N4O16 | |
| Molar mass | 836.795 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
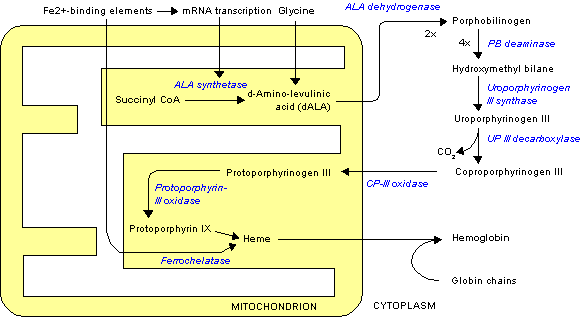
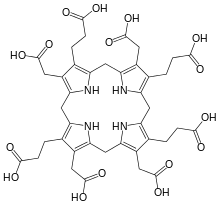
Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens.
Biosynthetic context
Biosynthesis
Its biosynthetic precursor is the linear tetrapyrrole hydroxymethylbilane, which is converted to uroporphyrinogen III by the action of uroporphyrinogen-III synthase.[1] If, however, uroporphyrinogen-III synthase is not present, the hydroxymethylbilane will spontaneously cyclise into uroporphyrinogen I. The difference between the uroporphyrinogen I and III is the arrangement of the four propionic acid ("P" groups) and the four acetic acid groups ("A" groups). Uroporphyrinogen I features in an AP-AP-AP-AP symmetry, whereas in uroporphyrinogen III one AP-group is reversed and hence an AP-AP-AP-PA arrangement.
Conversion to heme and sirohemes, etc.
In the biosynthesis of sirohemes, uroporphyrinogen III is converted by two methyl transferases to dihydrosirohydrochlorin, which is subsequently oxidized sirohydrochlorin, a precursor to the siroheme prosthetic group. In the biosynthesis of hemes and chlorophylls, uroporphyrinogen III is converted into coproporphyrinogen III by the enzyme uroporphyrinogen III decarboxylase.
See also
References
- ↑ Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221.