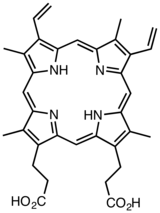
Protoporphyrin IX
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.213 |
PubChem CID |
|
| |
| |
| Properties | |
| C34H34N4O4 | |
| Molar mass | 562.658 g/mol |
| Density | 1.27 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Protoporphyrin IX is an organic compound, which is one of the most common porphyrins in nature. It is a deeply colored pigment that is not soluble in basic water. The free porphyrin is encountered in nature in the form of its iron complexes. When complexed with ferrous iron, the molecule is called heme. Hemes are prosthetic groups in some important proteins. These heme-containing proteins include hemoglobin, myoglobin, and cytochrome c. Complexes can also be formed with other metal ions, such as zinc.[1]
Protoporphyrin IX is a largely planar molecule. The N-H groups are trans.[2]
Biosynthesis
Its biosynthesis is mediated by the enzyme protoporphyrinogen oxidase. The simplified biosynthetic sequence is acyclic precursors → mono-pyrrole (porphobilinogen) → tetrapyrrole, also known as a porphyrinogen (uroporphyrinogen III) → porphyrin (protoporphyrin IX).[1]
Protoporphyrin IX is an important precursor to biologically essential prosthetic groups such as heme, cytochrome c, and chlorophylls. As a result, a number of organisms are able to synthesize these tetrapyrrole from basic precursors such as glycine and succinyl CoA, or glutamate. Despite the wide range of organisms that synthesize protoporphyrin IX the process is largely conserved from bacteria to mammals with a few distinct exceptions in higher plants.[3][4][5]
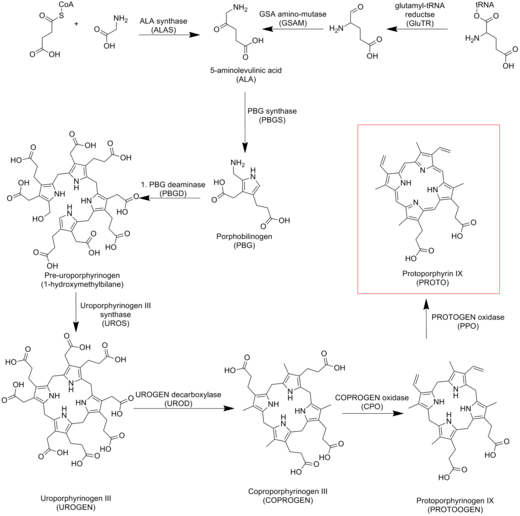
Heme b and chlorophyll biosyntheses
In the biosynthesis of biological cofactors, PPIX is metalated by the action of chelatases. Ferrochelatase converts PPIX into heme b (i.e. Fe-protoporphyrin IX or protoheme IX). In chlorophyll biosynthesis, the enzyme magnesium chelatase converts it into Mg-protoporphyrin IX.
Synthetic iron derivatives
Protoporphyrin IX reacts with iron salts in air to give the FeCl(PPIX).[6]
Protoporphyin nomenclature
Protoporphyrins are tetrapyrroles containing four methyl, two propionic acid, and two vinyl side chains. The number (e.g. IX) indicates the position of these side chains, but historically, as the nomenclature has grown, it has done so systematically only in parts.
See also
- 1 2 Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221.
- ↑ Winslow S. Caughey, James A. Ibers (1977). "Crystal and Molecular Structure of the Free Base Porphyrin, Protoporphyrin IX Dimethyl Ester". J. Am. Chem. Soc. 99: 6639–6645. doi:10.1021/ja00462a027.
- ↑ A. R. Battersby; C. J. R. Fookes; G. W. J. Matcham; E. McDonald (1980). "Biosynthesis of the pigments of life: formation of the macrocycle". Nature. 285: 17–21. doi:10.1038/285017a0.
- ↑ F. J. Leeper (1983). "The biosynthesis of porphyrins, chlorophylls, and vitamin B12". Natural Product Reports. 2: 19–47. doi:10.1039/NP9850200019.
- ↑ G. Layer; J. Reichelt; D. Jahn (2010). "Structure and function of enzymes in heme biosynthesis". Protein Science. 19: 1137–1161. doi:10.1002/pro.405. PMC 2895239.
- ↑ Chang, C. K.; DiNello, R. K.; Dolphin, D. (1980). "Iron Porphines". Inorg. Synth. Inorganic Syntheses. 20: 147. doi:10.1002/9780470132517.ch35.