Chlorin
 | |
| Names | |
|---|---|
| Other names
2,3-Dihydroporphine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C20H16N4 | |
| Molar mass | 312.36784 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
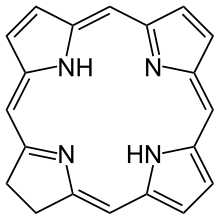
In organic chemistry, a chlorin is a large heterocyclic aromatic ring consisting, at the core, of three pyrroles and one pyrroline coupled through four =CH- linkages. Unlike porphin, the central aromatic ring structure of porphyrins, a chlorin is largely aromatic but not aromatic through the entire circumference of the ring.[1]
Magnesium-containing chlorins are called chlorophylls, and are the central photosensitive pigment in chloroplasts.
Related compounds, with two pyrroles and two pyrrolines (which are like pyrroles with one double bond reduced to a single bond) in the macrocycle are called bacteriochlorins and isobacteriochlorins.[2]

Because of their photosensitivity, chlorins are in active use as photosensitizing agents in experimental photodynamic therapy.[3]
See also
References
- ↑ Juse´lius, Jonas; Sundholm, Dage (2000). "The aromatic pathways of porphins, chlorins and bacteriochlorins" (PDF). Physical Chemistry Chemical Physics. 2 (10): 2145–2151. doi:10.1039/b000260g.
- ↑ "ChEBI". June 16, 2009. pp. isobacteriochlorin (CHEBI:52583). Retrieved 1 February 2012.
- ↑ Spikes, John D. (July 1990). "New trends in photobiology". Journal of Photochemistry and Photobiology B: Biology. 6 (3): 259–274. doi:10.1016/1011-1344(90)85096-F.