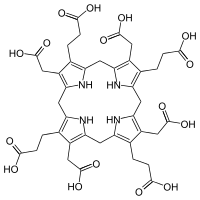
Uroporphyrinogen I
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C40H44N4O16 | |
| Molar mass | 836.795 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uroporphyrinogen I is an isomer of uroporphyrinogen III, a metabolic intermediate in the biosynthesis of heme. Uroporphyrinogen I is produced in lesser quantities in acute intermittent porphyria.
Biosynthetic context
The tetrapyrrole called hydroxymethylbilane is normally converted by the action of uroporphyrinogen-III synthase to uroporphyrinogen III.[1] Uroporphyrinogen III is subsequently converted into coproporphyrinogen III and on to heme. If, however, no uroporphyrinogen-III synthase is present, hydroxymethylbilane will spontaneously cyclise into uroporphyrinogen I, which is then converted into coproporphyrinogen I, also by uroporphyrinogen III decarboxylase.
The difference between the two forms, is the arrangements of the four carboxyethyl ("P" groups) and the four carboxymethyl groups ("A" groups). The non-enzyamtic conversion to uroporphyrinogen I results in the sequence AP-AP-AP-AP, whereas the enzymatic conversion into uroporphyrinogen III lead to reversal of one AP-group and hence an AP-AP-AP-PA arrangement.
References
- ↑ Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221.