Proteasome endopeptidase complex
| Proteasome endopeptidase complex | |||||||||
|---|---|---|---|---|---|---|---|---|---|
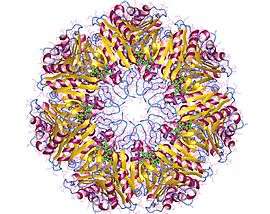 Proteasome core particle, di-heptamer, Archaea | |||||||||
| Identifiers | |||||||||
| EC number | 3.4.25.1 | ||||||||
| CAS number | 140879-24-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Proteasome endopeptidase complex (EC 3.4.25.1, ingensin, macropain, multicatalytic endopeptidase complex, prosome, multicatalytic proteinase (complex), MCP, proteasome, large multicatalytic protease, proteasome organelle, alkaline protease, 26S protease, tricorn proteinase, tricorn protease) is an enzyme.[1][2][3][4] This enzyme catalyses the following chemical reaction
- Cleavage of peptide bonds with very broad specificity
This 20-S protein is composed of 28 subunits arranged in four rings of seven.
References
- ↑ Seemüller E, Lupas A, Stock D, Löwe J, Huber R, Baumeister W (April 1995). "Proteasome from Thermoplasma acidophilum: a threonine protease". Science. 268 (5210): 579–82. doi:10.1126/science.7725107. PMID 7725107.
- ↑ Coux O, Tanaka K, Goldberg AL (1996). "Structure and functions of the 20S and 26S proteasomes". Annual Review of Biochemistry. 65: 801–47. doi:10.1146/annurev.bi.65.070196.004101. PMID 8811196.
- ↑ Groll M, Ditzel L, Löwe J, Stock D, Bochtler M, Bartunik HD, Huber R (April 1997). "Structure of 20S proteasome from yeast at 2.4 A resolution". Nature. 386 (6624): 463–71. doi:10.1038/386463a0. PMID 9087403.
- ↑ Dick TP, Nussbaum AK, Deeg M, Heinemeyer W, Groll M, Schirle M, Keilholz W, Stevanović S, Wolf DH, Huber R, Rammensee HG, Schild H (October 1998). "Contribution of proteasomal beta-subunits to the cleavage of peptide substrates analyzed with yeast mutants". The Journal of Biological Chemistry. 273 (40): 25637–46. doi:10.1074/jbc.273.40.25637. PMID 9748229.
External links
- Proteasome+endopeptidase+complex at the US National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.