METAP1
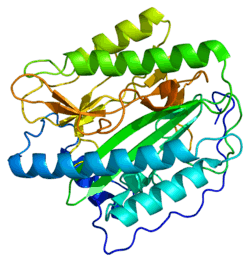
Methionine aminopeptidase 1 is an enzyme that in humans is encoded by the METAP1 gene.[5][6][7]
References
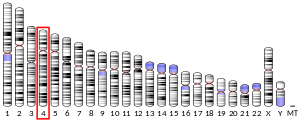
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000164024 - Ensembl, May 2017
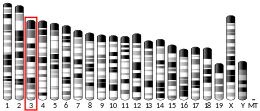
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000005813 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Nagase T, Miyajima N, Tanaka A, Sazuka T, Seki N, Sato S, Tabata S, Ishikawa K, Kawarabayasi Y, Kotani H, et al. (Jul 1995). "Prediction of the coding sequences of unidentified human genes. III. The coding sequences of 40 new genes (KIAA0081-KIAA0120) deduced by analysis of cDNA clones from human cell line KG-1". DNA Res. 2 (1): 37–43. doi:10.1093/dnares/2.1.37. PMID 7788527.
- ↑ Dummitt B, Fei Y, Chang YH (Jul 2002). "Functional expression of human methionine aminopeptidase type 1 in Saccharomyces cerevisiae". Protein Pept Lett. 9 (4): 295–303. doi:10.2174/0929866023408607. PMID 12144506.
- ↑ "Entrez Gene: METAP1 methionyl aminopeptidase 1".
Further reading
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Serero A, Giglione C, Sardini A, et al. (2004). "An unusual peptide deformylase features in the human mitochondrial N-terminal methionine excision pathway". J. Biol. Chem. 278 (52): 52953–63. doi:10.1074/jbc.M309770200. PMID 14532271.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Rush J, Moritz A, Lee KA, et al. (2005). "Immunoaffinity profiling of tyrosine phosphorylation in cancer cells". Nat. Biotechnol. 23 (1): 94–101. doi:10.1038/nbt1046. PMID 15592455.
- Andersen JS, Lam YW, Leung AK, et al. (2005). "Nucleolar proteome dynamics". Nature. 433 (7021): 77–83. doi:10.1038/nature03207. PMID 15635413.
- Addlagatta A, Hu X, Liu JO, Matthews BW (2006). "Structural basis for the functional differences between type I and type II human methionine aminopeptidases". Biochemistry. 44 (45): 14741–9. doi:10.1021/bi051691k. PMID 16274222.
- Kimura K, Wakamatsu A, Suzuki Y, et al. (2006). "Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes". Genome Res. 16 (1): 55–65. doi:10.1101/gr.4039406. PMC 1356129. PMID 16344560.
- Selvakumar P, Lakshmikuttyamma A, Dimmock JR, Sharma RK (2006). "Methionine aminopeptidase 2 and cancer". Biochim. Biophys. Acta. 1765 (2): 148–54. doi:10.1016/j.bbcan.2005.11.001. PMID 16386852.
- Hu X, Addlagatta A, Lu J, et al. (2007). "Elucidation of the function of type 1 human methionine aminopeptidase during cell cycle progression". Proc. Natl. Acad. Sci. U.S.A. 103 (48): 18148–53. doi:10.1073/pnas.0608389103. PMC 1838721. PMID 17114291.
- Ewing RM, Chu P, Elisma F, et al. (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Mol. Syst. Biol. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.