Tavilermide
 | |
| Clinical data | |
|---|---|
| Routes of administration | Eye drop |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
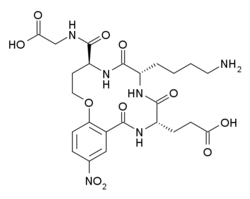
| Formula | C24H32N6O11 |
| Molar mass | 580.54448 g/mol |
| 3D model (JSmol) | |
| |
| |
Tavilermide (INN) (developmental code name MIM-D3) is a selective, cyclic peptide partial agonist of TrkA. (this class of drugs are sometimes known as nerve growth factor (NGF) mimetics)[1][2][3] It is under development by Mimetogen Pharmaceuticals as an ophthalmic (eye drop) solution for the treatment of dry eyes, and is in phase III clinical trials for this indication. Tavilermide is currently being evaluated in two multi-center phase III clinical studies in the United States for the treatment of dry eye disease. Tavilermide is also in phase I clinical trials for the treatment of glaucoma; studies are ongoing.[4][5]
See also
References
- ↑ Meerovitch, Karen; Torkildsen, Gail; Lonsdale, John; Goldfarb, Heidi; Lama, Teresa; Cumberlidge, Garth; Ousler III, George W. (2013). "Safety and efficacy of MIM D3 ophthalmic solutions in a randomized placebo controlled Phase 2 clinical trial in patients with dry eye". Clinical Ophthalmology: 1275. doi:10.2147/OPTH.S44688. ISSN 1177-5483.
- ↑ Jain, Pooja; Li, Ruihong; Lama, Teresa; Saragovi, H. Uri; Cumberlidge, Garth; Meerovitch, Karen (2011). "An NGF mimetic, MIM-D3, stimulates conjunctival cell glycoconjugate secretion and demonstrates therapeutic efficacy in a rat model of dry eye". Experimental Eye Research. 93 (4): 503–512. doi:10.1016/j.exer.2011.06.014. ISSN 0014-4835.
- ↑ Vickers, Laura A.; Gupta, Preeya K. (2015). "The Future of Dry Eye Treatment: A Glance into the Therapeutic Pipeline". Ophthalmology and Therapy. doi:10.1007/s40123-015-0038-y. ISSN 2193-8245. PMC 4675732.
- ↑ Chang EE, Goldberg JL (2012). "Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement". Ophthalmology. 119 (5): 979–86. doi:10.1016/j.ophtha.2011.11.003. PMC 3343191. PMID 22349567.
- ↑ "Tavilermide - AdisInsight". adisinsight.springer.com. Retrieved 18 August 2016.
External links
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.