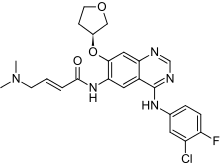
Afatinib
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Gilotrif, Giotrif |
| Synonyms | BIBW 2992 |
| AHFS/Drugs.com | Multum Consumer Information |
| License data | |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 95% |
| Metabolism | CYP not involved |
| Elimination half-life | 37 hours |
| Excretion | Faeces (85%), urine (4%) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.239.035 |
| Chemical and physical data | |
| Formula | C24H25ClFN5O3 |
| Molar mass | 485.937 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Afatinib, sold under the brand name Gilotrif among others, is a medication used to treat non-small cell lung carcinoma (NSCLC).[1][2][3] It belongs to the tyrosine kinase inhibitor family of medications.[4] It is taken by mouth.[4]
It is mainly used to treat cases of NSCLC that harbour mutations in the epidermal growth factor receptor (EGFR) gene.[5]
Medical uses
It has received regulatory approval for use as a treatment for non-small cell lung cancer,[6][4][7][8] although there is emerging evidence to support its use in other cancers such as breast cancer.[9]
Adverse effects
Adverse effects by frequency include:[6][4][7][8][10]
- Very common (>10% frequency)
- Diarrhea (>90%)
- Rash/dermatitis acneform
- Stomatitis
- Paronychia
- Decreased appetite
- Nose bleed
- Itchiness
- Dry skin
- Common (1–10% frequency)
- Dehydration
- Taste changes
- Dry eye
- Cystitis
- Cheilitis
- Fever
- Runny/stuffy nose
- Low amount of potassium in the blood
- Conjunctivitis
- Increased ALT
- Increased AST
- Hand-foot syndrome
- Muscle spasms
- Kidney impairment and/or failure
- Uncommon (0.1-1% frequency)
Mechanism of action
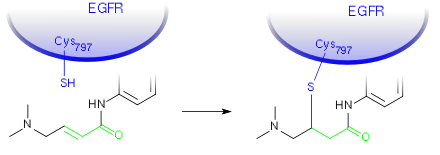
Like lapatinib and neratinib, afatinib is a protein kinase inhibitor that also irreversibly inhibits human epidermal growth factor receptor 2 (Her2) and epidermal growth factor receptor (EGFR) kinases. Afatinib is not only active against EGFR mutations targeted by first generation tyrosine-kinase inhibitors (TKIs) like erlotinib or gefitinib, but also against mutations such as T790M which are not sensitive to these standard therapies.[11] Because of its additional activity against Her2, it is being investigated for breast cancer as well as other EGFR and Her2 driven cancers.[2]
Clinical trials
In March 2010 a Phase III trial in NSCLC patients called Lux-Lung 5 began with this drug.[13] Fall 2010 interim results suggested the drug extended progression-free survival threefold compared to placebo, but did not extend overall survival.[14] In May 2012, the Phase IIb/III trial Lux-Lung 1 came to the same conclusion.[15]
In January 2015 a Phase III trial in people with NSCLC suggested the drug extended life expectancy in stage IV NSCLC adenocarcinoma with EGFR Mutation type del 19-positive tumors, compared to cisplatin-based chemotherapy by a year (33 months vs. 21 months).[16] It also shows strong activity against exon 18 mutations (particularly G719) and is currently the preferred EGFR-TKI therapy for exon 18 mutations (particularly G719x).[17]
Phase II results for breast cancer that over-expresses the protein human epidermal growth factor receptor 2 (Her2-positive breast cancer) were described as promising by the authors, with 19 of 41 patients achieving benefit from afatinib.[9] Double-blind Phase III trials are under way to confirm or refute this finding. Her2-negative breast cancers showed limited or no response to the drug.[18]
References
- ↑ H. Spreitzer (13 May 2008). "Neue Wirkstoffe – Tovok". Österreichische Apothekerzeitung (in German) (10/2008): 498.
- 1 2 Minkovsky N, Berezov A (December 2008). "BIBW-2992, a dual receptor tyrosine kinase inhibitor for the treatment of solid tumors". Curr Opin Investig Drugs. 9 (12): 1336–46. PMID 19037840.
- ↑ "Afatinib". US Food and Drug Administration. 12 July 2013.
- 1 2 3 4 "GIOTRIF® Afatinib (as afatinib dimaleate)" (PDF). TGA eBusiness Services. Boehringer Ingelheim Pty Limited. 7 November 2013. Retrieved 28 January 2014.
- ↑ Vavalà, T (2017). "Role of afatinib in the treatment of advanced lung squamous cell carcinoma". Clinical Pharmacology: Advances and Applications. 9: 147–157. doi:10.2147/CPAA.S112715. PMC 5709991. PMID 29225480.
- 1 2 "GILOTRIF (afatinib) tablet, film coated [Boehringer Ingelheim Pharmaceuticals, Inc.]". DailyMed. Boehringer Ingelheim Pharmaceuticals, Inc. November 2013. Retrieved 28 January 2014.
- 1 2 "Giotrif 20 mg film-coated tablets – Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Boehringer Ingelheim Limited. 20 January 2014. Retrieved 28 January 2014.
- 1 2 "Giotrif : EPAR -Product Information" (PDF). European Medicines Agency. Boehringer Ingelheim International GmbH. 16 October 2013. Retrieved 28 January 2014.
- 1 2 Lin NU, Winer EP, Wheatley D, Carey LA, Houston S, Mendelson D, Munster P, Frakes L, Kelly S, Garcia AA, Cleator S, Uttenreuther-Fischer M, Jones H, Wind S, Vinisko R, Hickish T (2012). "A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab". Breast Cancer Research and Treatment. 133 (3): 1057–65. doi:10.1007/s10549-012-2003-y. PMC 3387495. PMID 22418700.
- ↑ "Gilotrif (afatinib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 28 January 2014.
- ↑ Li (2008). "BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models". Oncogene. 27 (34): 4702–4711. doi:10.1038/onc.2008.109. PMC 2748240. PMID 18408761.
- ↑ Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel Frühjahr 2013. (in German)
- ↑ Clinical trial number NCT01085136 for "LUX-Lung 5: BIBW 2992 Plus Weekly Paclitaxel Versus Investigator's Choice of Single Agent Chemotherapy Following BIBW 2992 Monotherapy in Non-small Cell Lung Cancer Patients Failing Erlotinib or Gefitinib" at ClinicalTrials.gov
- ↑ "Afatinib (BIBW 2992*) Triples Progression Free Survival in Phase III Study in Lung Cancer Patients". BusinessWire. 11 October 2010.
- ↑ Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, Zhou C, Su WC, Wang M, Sun Y, Heo DS, Crino L, Tan EH, Chao TY, Shahidi M, Cong XJ, Lorence RM, Yang JC (2012). "Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial". The Lancet Oncology. 13 (5): 528–38. doi:10.1016/S1470-2045(12)70087-6. PMID 22452896.
- ↑ Yang, JC; Wu, YL; Schuler, M; Sebastian, M; Popat, S; Yamamoto, N; Zhou, C; Hu, CP; O'Byrne, K; Feng, J; Lu, S; Huang, Y; Geater, SL; Lee, KY; Tsai, CM; Gorbunova, V; Hirsh, V; Bennouna, J; Orlov, S; Mok, T; Boyer, M; Su, WC; Lee, KH; Kato, T; Massey, D; Shahidi, M; Zazulina, V; Sequist, LV (February 2015). "Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials". The Lancet Oncology. 16 (2): 141–51. doi:10.1016/s1470-2045(14)71173-8. PMID 25589191.
- ↑ Kobayashi, Y (2015). "EGFR Exon 18 Mutations in Lung Cancer: Molecular Predictors of Augmented Sensitivity to Afatinib or Neratinib as Compared with First- or Third-Generation TKIs". Clin Cancer Res. 21 (23): 5305–13. doi:10.1158/1078-0432.CCR-15-1046. PMID 26206867.
- ↑ Schuler M, Awada A, Harter P, Canon JL, Possinger K, Schmidt M, De Grève J, Neven P, Dirix L, Jonat W, Beckmann MW, Schütte J, Fasching PA, Gottschalk N, Besse-Hammer T, Fleischer F, Wind S, Uttenreuther-Fischer M, Piccart M, Harbeck N (2012). "A phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2-negative metastatic breast cancer". Breast Cancer Research and Treatment. 134 (3): 1149–59. doi:10.1007/s10549-012-2126-1. PMC 3409367. PMID 22763464.