Terpene

Terpenes (/ˈtɜːrpiːn/) are a large and diverse class of organic compounds, produced by a variety of plants, particularly conifers, and by some insects.[1][2] They often have a strong odor and may protect the plants that produce them by deterring herbivores and by attracting predators and parasites of herbivores.[3][4] Although sometimes used interchangeably with "terpenes", terpenoids (or isoprenoids) are modified terpenes as they contain additional functional groups, usually oxygen-containing.[5] Terpenes are hydrocarbons.
Terpenes are the major components of rosin and of turpentine produced from resin. The name "terpene" is derived from the word "terpentine", an obsolete form of the word "turpentine". Terpenes are also major biosynthetic building blocks. Steroids, for example, are derivatives of the triterpene squalene.
Terpenes and terpenoids are the primary constituents of the essential oils of many types of medicinal plants and flowers. Essential oils are used widely as fragrances in perfumery, and in medicine and alternative medicines such as aromatherapy. Synthetic variations and derivatives of natural terpenes and terpenoids also greatly expand the variety of aromas used in perfumery and flavors used in food additives. Vitamin A is a terpenoid.
Structure and biosynthesis
Isoprene phase
Terpenes are derived biosynthetically from units of isopentenyl pyrophosphate. Although the structures of terpenoids are rationalized as derivatives of isoprene (2-methyl-1,3-butadiene), isoprene is not involved in the biosynthesis. The biogenetic isoprene rule or the C5 rule was described in 1953, Leopold Ružička who explained that terpinoids can be visualized as the result of linking isoprene units "head to tail" to form chains, which can be arranged to form rings.[6]
There are two metabolic pathways that create terpenoids:
Mevalonic acid pathway
Many organisms manufacture terpenoids through the HMG-CoA reductase pathway, known as the Mevalonate pathway, which also produces cholesterol. One of the intermediates in this pathway is mevalonic acid. The reactions take place in the cytosol. The pathway was discovered in the 1950s.
MEP/DOXP pathway
The 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate pathway (MEP/DOXP pathway), also known as non-mevalonate pathway or mevalonic acid-independent pathway, takes place in the plastids of plants and apicomplexan protozoa, as well as in many bacteria. It was discovered in the late 1980s.
Pyruvate and glyceraldehyde 3-phosphate are converted by DOXP synthase (Dxs) to 1-deoxy-D-xylulose 5-phosphate, and by DOXP reductase (Dxr, IspC) to 2-C-methyl-D-erythritol 4-phosphate (MEP). The subsequent three reaction steps catalyzed by 4-diphosphocytidyl-2-C-methyl-D-erythritol synthase (YgbP, IspD), 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase (YchB, IspE), and 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase (YgbB, IspF) mediate the formation of 2-C-methyl-D-erythritol 2,4-cyclopyrophosphate (MEcPP). Finally, MEcPP is converted to (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by HMB-PP synthase (GcpE, IspG), and HMB-PP is converted to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) by HMB-PP reductase (LytB, IspH).
IPP and DMAPP are the end-products in either pathway, and are the precursors of isoprene, monoterpenoids (10-carbon), diterpenoids (20-carbon), carotenoids (40-carbon), chlorophylls, and plastoquinone-9 (45-carbon). Synthesis of all higher terpenoids proceeds via formation of geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP), and geranylgeranyl pyrophosphate (GGPP).
The MVA and MEP are mutually exclusive in most organisms.
| Organism | Pathways |
|---|---|
| Bacteria | MVA or MEP |
| Archaea | MVA |
| Green Algae | MEP |
| Plants | MVA and MEP |
| Animals | MVA |
| Fungi | MVA |
Geranyl pyrophosphate phase and beyond
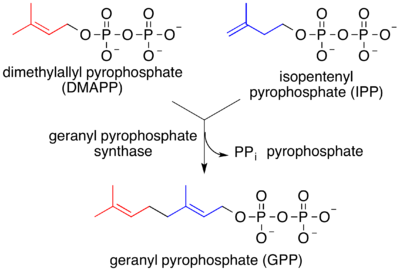
In both MVA and MEP pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. IPP and DMAPP condense to give geranyl pyrophosphate, the precursor to monoterpenes and monoterpenoids.
Geranyl pyrophosphate is also converted to farnesyl pyrophosphate and geranylgeranyl pyrophosphate, respectively C15 and C20 precursors to sesquiterpenes and diterpenes (as well as sesequiterpenoids and diterpenoids).[2] Biosynthesis is mediated by terpene synthase.[7][8]
Types
- Selected terpenes
 Limonene, a monoterpene.
Limonene, a monoterpene. Carvone is a monoterpenoid, a modified monoterpene.
Carvone is a monoterpenoid, a modified monoterpene.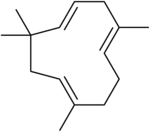 Humulene, a sesquiterpene.
Humulene, a sesquiterpene.

Terpenes may be classified by the number of isoprene units in the molecule; a prefix in the name indicates the number of terpene units needed to assemble the molecule.
- Hemiterpenes consist of a single isoprene unit. Isoprene itself is considered the only hemiterpene, but oxygen-containing derivatives such as prenol and isovaleric acid are hemiterpenoids.
- Monoterpenes consist of two isoprene units and have the molecular formula C10H16. Examples of monoterpenes and monoterpenoids include geraniol, terpineol (present in lilacs), limonene (present in citrus fruits), myrcene (present in hops), linalool (present in lavender) or pinene (present in pine trees).[9] Iridoids derive from monoterpenes.
- Sesquiterpenes consist of three isoprene units and have the molecular formula C15H24. Examples of sesquiterpenes and sesquiterpenoids include humulene, farnesenes, farnesol. (The sesqui- prefix means one and a half.)
- Diterpenes are composed of four isoprene units and have the molecular formula C20H32. They derive from geranylgeranyl pyrophosphate. Examples of diterpenes and diterpenoids are cafestol, kahweol, cembrene and taxadiene (precursor of taxol). Diterpenes also form the basis for biologically important compounds such as retinol, retinal, and phytol.
- Sesterterpenes, terpenes having 25 carbons and five isoprene units, are rare relative to the other sizes. (The sester- prefix means two and a half.) An example of a sesterterpenoid is geranylfarnesol.
- Triterpenes consist of six isoprene units and have the molecular formula C30H48. The linear triterpene squalene, the major constituent of shark liver oil, is derived from the reductive coupling of two molecules of farnesyl pyrophosphate. Squalene is then processed biosynthetically to generate either lanosterol or cycloartenol, the structural precursors to all the steroids.
- Sesquarterpenes are composed of seven isoprene units and have the molecular formula C35H56. Sesquarterpenes are typically microbial in their origin. Examples of sesquarterpenoids are ferrugicadiol and tetraprenylcurcumene.
- Tetraterpenes contain eight isoprene units and have the molecular formula C40H64. Biologically important tetraterpenoids include the acyclic lycopene, the monocyclic gamma-carotene, and the bicyclic alpha- and beta-carotenes.
- Polyterpenes consist of long chains of many isoprene units. Natural rubber consists of polyisoprene in which the double bonds are cis. Some plants produce a polyisoprene with trans double bonds, known as gutta-percha.
- Norisoprenoids, such as the C13-norisoprenoids 3-oxo-α-ionol present in Muscat of Alexandria leaves and 7,8-dihydroionone derivatives, such as megastigmane-3,9-diol and 3-oxo-7,8-dihydro-α-ionol found in Shiraz leaves (both grapes in the species Vitis vinifera)[10] or wine[11][12] (responsible for some of the spice notes in Chardonnay), can be produced by fungal peroxidases[13] or glycosidases.[14]
Properties
Terpenes have desirable properties for use in food, cosmetics, pharmaceutical and biotechnology industries.[15][16]
The genomes of 17 plant species contain genes that encode terpenoid synthase enzymes imparting terpenes with their basic structure, and cytochrome P450s that modify this basic structure.[17]
Terpenes are useful active ingredients as part of natural agricultural pesticides.[18] Terpenes are used by termites of the subfamily Nasutitermitinae to ward off predatory insects, through the use of a specialized mechanism called a fontanellar gun.[19]
Higher amounts of terpenes are released by trees in warmer weather. In fact they are proposed to act as a natural form of cloud seeding. The clouds reflect sunlight, allowing the forest temperature to regulate.[20] The aroma and flavor of hops comes, in part, from sesquiterpenes (mainly α-humulene and β-caryophyllene), which affect beer quality.[21] Terpenes are also major constituents of Cannabis sativa plants, which contain at least 120 identified compounds.[22]
Industrial syntheses
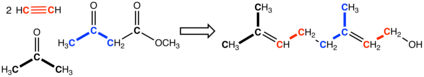
While terpenes and terpenoids occur widely, their extraction from natural sources is often problematic. Consequently, they are produced by chemical synthesis, usually from petrochemicals. In one route, acetone and acetylene are condensed to give 2-Methylbut-3-yn-2-ol, which is extended with acetoacetic ester to give geranyl alcohol. Others are prepared from those terpenes and terpenoids that are readily isolated in quantity, say from the paper and tall oil industries. For example, α-pinene, which is really obtainable from natural sources, is converted to citronellal and camphor. Citronellal is also converted to rose oxide and menthol.[1]
References
- 1 2 Eberhard Breitmaier (2006). Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. Wiley-VCH. doi:10.1002/9783527609949. ISBN 9783527609949.
- 1 2 Davis, Edward M.; Croteau, Rodney (2000). "Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes". Topics in Current Chemistry. 209: 53–95. doi:10.1007/3-540-48146-X_2.
- ↑ Martin, D. M.; Gershenzon, J.; Bohlmann, J. (July 2003). "Induction of Volatile Terpene Biosynthesis and Diurnal Emission by Methyl Jasmonate in Foliage of Norway Spruce". Plant Physiology. 132 (3): 1586–1599. doi:10.1104/pp.103.021196. PMC 167096. Retrieved 1 November 2014.
- ↑ Pichersky, E. (10 February 2006). "Biosynthesis of Plant Volatiles: Nature's Diversity and Ingenuity". Science. 311 (5762): 808–811. Bibcode:2006Sci...311..808P. doi:10.1126/science.1118510. PMC 2861909. PMID 16469917.
- ↑ https://goldbook.iupac.org/html/T/T06279.html
- ↑ Ružička, Leopold (1953). "The isoprene rule and the Biogenesis of terpenic compounds". Cellular and Molecular Life Sciences. 9 (10): 357–367. doi:10.1007/BF02167631.
- ↑ Kumari, I.; Ahmed, M.; Akhter, Y. (2017). "Evolution of catalytic microenvironment governs substrate and product diversity in trichodiene synthase and other terpene fold enzymes". Biochimie. 144: 9. doi:10.1016/j.biochi.2017.10.003. PMID 29017925.
- ↑ Pazouki, L.; Niinemets, Ü. (2016). "Multi-Substrate Terpene Synthases: Their Occurrence and Physiological Significance". Frontiers in Plant Science. 7: 1019. doi:10.3389/fpls.2016.01019. PMC 4940680. PMID 27462341.
- ↑ Breitmaier, Eberhard (2006). Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. John Wiley & Sons. pp. 1–13. ISBN 3527317864.
- ↑ Günata, Z.; Wirth, J. L.; Guo, W.; Baumes, R. L. (2001). "Carotenoid-Derived Aroma Compounds; chapter 13: Norisoprenoid Aglycon Composition of Leaves and Grape Berries from Muscat of Alexandria and Shiraz Cultivars". ACS Symposium Series. ACS Symposium Series. 802: 255–261. doi:10.1021/bk-2002-0802.ch018. ISBN 0-8412-3729-8.
- ↑ Winterhalter, P.; Sefton, M. A.; Williams, P. J. (1990). "Volatile C13-Norisoprenoid Compounds in Riesling Wine Are Generated From Multiple Precursors". American Journal of Enology and Viticulture. 41 (4): 277–283.
- ↑ Vinholes, J.; Coimbra, M. A.; Rocha, S. M. (2009). "Rapid tool for assessment of C13 norisoprenoids in wines". Journal of Chromatography A. 1216 (47): 8398–8403. doi:10.1016/j.chroma.2009.09.061. PMID 19828152.
- ↑ Zelena, K.; Hardebusch, B.; Hülsdau, B.; Berger, R. G.; Zorn, H. (2009). "Generation of Norisoprenoid Flavors from Carotenoids by Fungal Peroxidases". Journal of Agricultural and Food Chemistry. 57 (21): 9951–9955. doi:10.1021/jf901438m. PMID 19817422.
- ↑ Cabaroğlu, T.; Selli, S.; Canbaş, A.; Lepoutre, J.-P.; Günata, Z. (2003). "Wine flavor enhancement through the use of exogenous fungal glycosidases". Enzyme and Microbial Technology. 33 (5): 581–587. doi:10.1016/S0141-0229(03)00179-0.
- ↑ Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. (2014). "Triterpene biosynthesis in plants". Annual Review of Plant Biology. 65: 225–257. doi:10.1146/annurev-arplant-050312-120229. PMID 24498976.
- ↑ Augustin, J. M.; Kuzina, V.; Andersen, S. B.; Bak, S. (2011). "Molecular activities, biosynthesis and evolution of triterpenoid saponins". Phytochemistry. 72 (6): 435–457. doi:10.1016/j.phytochem.2011.01.015. PMID 21333312. Retrieved 19 January 2015.
- ↑ Boutanaev, A. M.; Moses, T.; Zi, J.; Nelson, D. R.; Mugford, S. T.; Peters, R. J.; Osbourn, A. (2015). "Investigation of terpene diversification across multiple sequenced plant genomes". Proceedings of the National Academy of Sciences. 112 (1): E81–E88. Bibcode:2015PNAS..112E..81B. doi:10.1073/pnas.1419547112. PMC 4291660. PMID 25502595.
- ↑ Isman, M. B. (2000). "Plant essential oils for pest and disease management". Crop Protection. 19 (8–10): 603–608. doi:10.1016/S0261-2194(00)00079-X. Retrieved 18 September 2014.
- ↑ Nutting, W. L.; Blum, M. S.; Fales, H. M. (1974). "Behavior of the North American Termite, Tenuirostritermes tenuirostris, with Special Reference to the Soldier Frontal Gland Secretion, Its Chemical Composition, and Use in Defense". Psyche. Hindawi Publishing Corporation. 81 (1): 167–177. doi:10.1155/1974/13854. Retrieved 22 July 2011.
- ↑ Adam, David (October 31, 2008). "Scientists discover cloud-thickening chemicals in trees that could offer a new weapon in the fight against global warming". The Guardian.
- ↑ Steenackers, B.; De Cooman, L.; De Vos, D. (2015). "Chemical transformations of characteristic hop secondary metabolites in relation to beer properties and the brewing process: A review". Food Chemistry. 172: 742–756. doi:10.1016/j.foodchem.2014.09.139. PMID 25442616.
- ↑ André, C. M.; Hausman, J.-F.; Guerriero, G. (2016). "Cannabis sativa: The Plant of the Thousand and One Molecules". Frontiers in Plant Science. 7: 19. doi:10.3389/fpls.2016.00019. PMC 4740396. PMID 26870049.
External links
| Wikimedia Commons has media related to Terpenes. |
- Institute of Chemistry - terpenes
- Structures of alpha pinene and beta pinene
- Terpenes at the US National Library of Medicine Medical Subject Headings (MeSH)