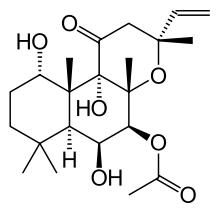
Forskolin
 | |
| Names | |
|---|---|
| IUPAC name
(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6,10,10b-Trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-3-vinyldodecahydro-1H-benzo[f]chromen-5-yl acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.060.354 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C22H34O7 | |
| Molar mass | 410.51 g·mol−1 |
| Solubility | Soluble in organic solvents such as ethanol, chloroform and DMSO[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Forskolin (coleonol) is a labdane diterpene that is produced by the Indian Coleus plant (Plectranthus barbatus). Other names include pashanabhedi, Indian coleus, makandi, HL-362, NKH477, and mao hou qiao rui hua.[2] As with other members of the large diterpene family of natural products, forskolin is derived from geranylgeranyl pyrophosphate (GGPP). Forskolin contains some unique functional elements, including the presence of a tetrahydropyran-derived heterocyclic ring. Forskolin is a commonly used material in laboratory research to increase levels of cyclic AMP by stimulation of adenylate cyclase.[2]
Cyclic AMP
Forskolin is commonly used as a tool in biochemistry to raise levels of cyclic AMP (cAMP) in the study and research of cell physiology.[2][3] Forskolin activates the enzyme adenylyl cyclase and increases intracellular levels of cAMP. cAMP is an important second messenger necessary for the proper biological response of cells to hormones and other extracellular signals. It is required for cell communication in the hypothalamus/pituitary gland axis and for the feedback control of hormones via induction of corticotropin-releasing factor gene transcription.[4] Cyclic AMP acts by activating cAMP-sensitive pathways such as protein kinase A and Epac.
Biosynthesis
The heterocyclic ring is synthesized after the formation of the trans-fused carbon ring systems formed by a carbocation mediated cyclization. The remaining tertiary carbocation is quenched by a molecule of water. After deprotonation, the remaining hydroxy group is free to form the heterocyclic ring. This cyclization can occur either by attack of the alcohol oxygen onto the allylic carbocation formed by loss of diphosphate, or by an analogous SN2' like displacement of the diphosphate.[5] This forms the core ring system A of forskolin.
The remaining modifications of the core ring system A can putatively be understood as a series of oxidation reactions to form a poly-ol B which is then further oxidized and esterified to form the ketone and acetate ester moieties seen in forskolin. However, because the biosynthetic gene cluster has not been described, this putative synthesis could be incorrect in the sequence of oxidation/esterification events, which could occur in almost any order.
Other uses
Other than its utility for laboratory research, forskolin has been used in traditional medicine in the belief it affects various disorders, and has been proposed as a weight loss agent, but none of these uses is supported by sound clinical evidence.[2]
See also
References
- ↑ "Forskolin" (pdf). Sigma Aldrich.
- 1 2 3 4 "Forskolin". Drugs.com. 2018. Retrieved 23 March 2018.
- ↑ Alasbahi, RH; Melzig, MF (January 2012). "Forskolin and derivatives as tools for studying the role of cAMP". Die Pharmazie. 67 (1): 5–13. PMID 22393824.
- ↑ Kageyama, K; Tamasawa, N; Suda, T (July 2011). "Signal transduction in the hypothalamic corticotropin-releasing factor system and its clinical implications". Stress (Amsterdam, Netherlands). 14 (4): 357–67. doi:10.3109/10253890.2010.536279. PMID 21438777.
- ↑ Dewick, P. M. (2009). Medicinal Natural Products (3rd ed.). Wiley. p. 232. ISBN 978-0470741689.
External links
- Hall, Harriet (2014-06-03). "Forskolin: Here We Go Again". Science-Based Medicine. Retrieved 2017-04-12.