Bisabolol
 | |
| Names | |
|---|---|
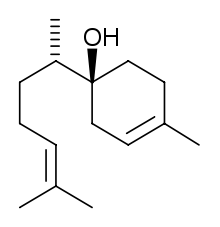
| IUPAC name
6-methyl-2-(4-methylcyclohex-3-en-1-yl)hept-5-en-2-ol | |
| Other names
Levomenol | |
| Identifiers | |
3D model (JSmol) |
|
| 5733954 | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.041.279 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.37 g·mol−1 |
| Density | 0.92 g cm−3 |
| Boiling point | 153 °C (307 °F; 426 K) at 12 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bisabolol, or more formally α-(−)-bisabolol or also known as levomenol,[1] is a natural monocyclic sesquiterpene alcohol. It is a colorless viscous oil that is the primary constituent of the essential oil from German chamomile (Matricaria recutita) and Myoporum crassifolium.[2] It is poorly soluble in water and glycerin, but soluble in ethanol.[3] The enantiomer, α-(+)-bisabolol, is also found naturally but is rare. Synthetic bisabolol is usually a racemic mixture of the two, α-(±)-bisabolol.
Bisabolol has a weak sweet floral aroma and is used in various fragrances. It has also been used for hundreds of years in cosmetics because of its perceived skin healing properties. Bisabolol is known to have anti-irritant, anti-inflammatory, and anti-microbial properties. Bisabolol is also demonstrated to enhance the percutaneous absorption of certain molecules.[4]
A structurally related compound known as β-bisabolol (CAS registry number [15352-77-9]) differs only in the position of the tertiary alcohol functional group.
References
- ↑ Rohstoff-Lexikon Bisabolol Archived February 20, 2008, at the Wayback Machine.
- ↑ Bisabolol (in english) Archived October 10, 2007, at the Wayback Machine.
- ↑ M. Eggersdorfer (2005). "Terpenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_205.
- ↑ Kamatou, Guy P. P.; Viljoen, Alvaro M. (2010). "A Review of the Application and Pharmacological Properties of α-Bisabolol and α-Bisabolol-Rich Oils" (PDF). Journal of the American Oil Chemists' Society. 87 (1): 1–7. doi:10.1007/s11746-009-1483-3.