Receptor-mediated endocytosis
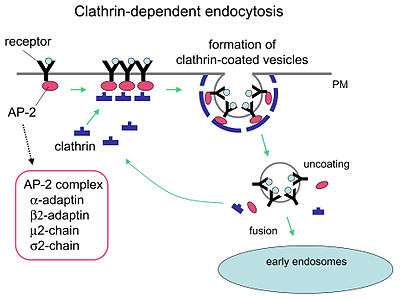
Receptor-mediated endocytosis (RME), also called clathrin-mediated endocytosis, is a process by which cells absorb metabolites, hormones, proteins – and in some cases viruses – by the inward budding of the plasma membrane (invagination). This process forms vesicles containing the absorbed substances and is strictly mediated by receptors on the surface of the cell. Only the receptor-specific substances can enter the cell through this process.
Process
Although receptors and their ligands can be brought into the cell through a few mechanisms (e.g. caveolin), clathrin-mediated endocytosis remains the best studied. Clathrin-mediated endocytosis of many receptor types begins with the cargo ligands in the luminal compartment of the cell binding to receptors on the cell membrane. The cargo ligand and receptor will then recruit adaptor proteins and clathrin triskelions to the outside membrane of the cell around where budding will form. Budding of the plasma membrane then occurs, forming a clathrin coated pit.[1] Other receptors can nucleate a clathrin-coated pit allowing formation around the receptor. A mature pit will be cleaved from the plasma membrane through the use of membrane binding and fission proteins such as dynamin (as well as other BAR domain proteins),[2] forming a clathrin-coated vesicle that then uncoats and typically fuses to an early endosome. Once fused, the endocytosed cargo (receptor and/or ligand) can then be sorted to lysosomal, recycling, or other trafficking pathways.[1]
Function
The function of receptor-mediated endocytosis is diverse. It is widely used for the specific uptake of certain substances required by the cell (examples include LDL via the LDL receptor or iron via transferrin). The role of receptor-mediated endocytosis is well recognized up take downregulation of transmembrane signal transduction but can also promote sustained signal transduction.[3] The activated receptor becomes internalised and is transported to late endosomes and lysosomes for degradation. However, receptor-mediated endocytosis is also actively implicated in transducing signals from the cell periphery to the nucleus. This became apparent when it was found that the association and formation of specific signaling complexes is required for the effective signaling of hormones (e.g. EGF). Additionally it has been proposed that the directed transport of active signaling complexes to the nucleus might be required to enable signaling as random diffusion is too slow[4] and mechanisms permanently downregulating incoming signals are strong enough to shut down signaling completely without additional signal-transducing mechanisms.[5]
Experiments
Using fluorescent dyes to stain the plasma membrane, it is possible to follow the internalization of plasma membrane fragments by microscopy.
Since the process is non-specific, the ligand can be a carrier for larger molecules. If the target cell has a known specific pinocytotic receptor, drugs can be attached and will be internalized.
To achieve internalisation of nanoparticles into cells, such as T cells, antibodies can be used to target the nanoparticles to specific receptors on the cell surface (such as CCR5).[6] This is one method of improving drug delivery to immune cells.
Characteristics
- Induction within minutes of exposure to excess ligand.
- The formation of these vesicles is sensitive to inhibition by wortmannin
- The initiation of vesicle formation can be delayed/inhibited by temperature variations
See also
References
- 1 2 Sorkin, Alexander; Puthenveedu, Manojkumar A. (2013-01-01). Yarden, Yosef; Tarcic, Gabi, eds. Clathrin-Mediated Endocytosis. Springer New York. pp. 1–31. doi:10.1007/978-1-4614-6528-7_1. ISBN 978-1-4614-6527-0.
- ↑ Kaksonen, Marko (Feb 2018). "Mechanisms of Clathrin mediated endocytosis". Nature.
- ↑ Thomsen AR, Plouffe B, Cahill TJ, Shukla AK, Tarrasch JT, Dosey AM, Kahsai AW, Strachan RT, Pani B, Mahoney JP, Huang L, Breton B, Heydenreich FM, Sunahara RK, Skiniotis G, Bouvier M, Lefkowitz RJ (2016). "GPCR-G Protein-β-Arrestin Super-Complex Mediates Sustained G Protein Signaling". Cell. 166: 907–19. doi:10.1016/j.cell.2016.07.004. PMID 27499021.
- ↑ Howe, Charles L. (2005). "Modeling the Signaling Endosome Hypothesis: Why a Drive to the Nucleus Is Better Than a (Random) Walk". Theor. Biol. Med. Mod. 2 (1): 43. doi:10.1186/1742-4682-2-43. PMC 1276819. PMID 16236165. 2:43.
- ↑ Kholodenko, Boris N. (2003). "Four-Dimensional Organisation of Protein Kinase Signaling Cascades: the Roles of Diffusion, Endocytosis and Molecular Motors". J. Exp. Biol. 206 (Pt 12): 2073–82. doi:10.1242/jeb.00298. PMID 12756289. 206, 2073-2082.
- ↑ Glass, Joshua (2016). "Human immune cell targeting of protein nanoparticles - caveospheres". Nanoscale. 8 (15): 8255–8265. doi:10.1039/C6NR00506C.
External links
- CytoChemistry.net- A lecture on RME with some nice pictures