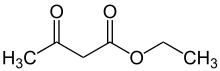
Ethyl acetoacetate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Ethyl 3-oxobutanoate | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.015 |
| KEGG | |
| RTECS number | AK5250000 |
| UNII | |
| |
| |
| Properties | |
| C6H10O3 | |
| Molar mass | 130.14 g/mol |
| Appearance | Colourless liquid |
| Odor | Fruit or rum |
| Density | 1.021 g/cm3, liquid |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 180.8 °C (357.4 °F; 453.9 K) |
| 2.86 g/100 ml (20 °C) | |
| Acidity (pKa) |
|
| −71.67×10−6 cm3/mol | |
| Hazards | |
EU classification (DSD) (outdated) |
Not listed |
| NFPA 704 | |
| Flash point | 70 °C (158 °F; 343 K) |
| Related compounds | |
Related esters |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is mainly used as a chemical intermediate in the production of a wide variety of compounds, such as amino acids, analgesics, antibiotics, antimalarial agents, antipyrine and aminopyrine, and vitamin B1; as well as the manufacture of dyes, inks, lacquers, perfumes, plastics, and yellow paint pigments. Alone, it is used as a flavoring for food.
Preparation
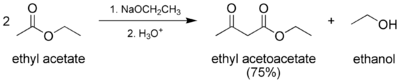
Ethyl acetoacetate is produced industrially by treatment of diketene with ethanol.[1]
The preparation of ethyl acetoacetate is a classic laboratory procedure.[2] It is prepared via the Claisen condensation of ethyl acetate. Two moles of ethyl acetate condense to form one mole each of ethyl acetoacetate and ethanol.
Reactivity
Ethyl acetoacetate is subject to keto-enol tautomerism. In the neat liquid at 33 °C, the enol consists of 15% of the total.[3]
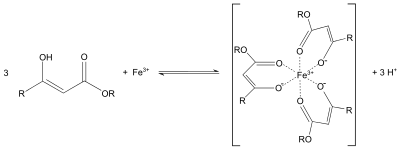
Ethyl acetoacetate is often used in the acetoacetic ester synthesis similar to diethyl malonate in the malonic ester synthesis or the Knoevenagel condensation. The protons alpha to carbonyl groups are acidic, and the resulting carbanion can undergo nucleophilic substitution. A subsequent thermal decarboxylation is also possible.[4] Similar to the behavior of acetylacetone, the enolate of ethyl acetoacetate can also serve as a bidentate ligand. For example, it forms purple coordination complexes with iron(III) salts:
Ethyl acetoacetate can also be reduced to ethyl 3-hydroxybutyrate.
Ethyl acetoacetate, when heated alone (uncatalyzed) with benzyl alcohol, forms synthetically useful benzyl acetoacetate (benzyl groups being easily removed later by catalytic hydrogenolysis over Pd/C under neutral conditions), via a mechanism involving acetylketene. Ethyl (and other) acetoacetates nitrosate readily with equimolar sodium nitrite in acetic acid, to afford the corresponding oximinoacetoacetate esters. A dissolving-zinc reduction of these in acetic acid in the presence of ketoesters or beta-diketones constitute the Knorr synthesis of pyrroles, useful for porphyrin synthesis.
See also
- Fructone, the ethylene glycol ketal of ethyl acetoacetate, an aroma compound
References
- ↑ Wilhelm Riemenschneider and Hermann M. Bolt "Esters, Organic" Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a09_565.pub2
- ↑ J. K. H. Inglis and K. C. Roberts (1926). "Ethyl Acetoacetate". Organic Syntheses. ; Collective Volume, 1, p. 235
- ↑ Jane L. Burdett; Max T. Rogers (1964). "Keto-Enol Tautomerism in β-Dicarbonyls Studied by Nuclear Magnetic Resonance Spectroscopy. I. Proton Chemical Shifts and Equilibrium Constants of Pure Compounds". J. Am. Chem. Soc. 86: 2105–2109. doi:10.1021/ja01065a003.
- ↑ Carey, Francis A. (2006). Organic Chemistry (Sixth ed.). New York, NY: McGraw-Hill. ISBN 0-07-111562-5.