Semaglutide
 | |
| Clinical data | |
|---|---|
| Trade names | Ozempic |
| AHFS/Drugs.com | ozempic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 89% |
| Metabolism | Proteolysis |
| Elimination half-life | 1 week |
| Duration of action | 63.6 h |
| Excretion | Urine and faeces |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
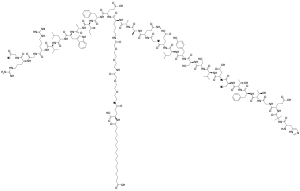
| Formula | C187H291N45O59 |
| Molar mass | 4,113.64 g·mol−1 |
Semaglutide (USAN; trade name Ozempic) is a pharmaceutical drug developed by Danish company Novo Nordisk for the treatment of type 2 diabetes.[1][2] It is marketed by the name Ozempic. As a glucagon-like peptide-1 receptor agonist, it lowers the blood sugar level by increasing the production of insulin. It was discovered in 2012,[3] by a team of researchers at Novo Nordisk as a longer-acting alternative to liraglutide.[4] Clinical trials were started in 2015, and phase 3 was completed in 2016.[5]
Researchers at the University of Leeds and Novo Nordisk reported in 2017 that it can also be used for the treatment of obesity.[6] It reduces hunger, food craving and body fat.[7]
FDA approval was applied in December 2016, and in October 2017 FDA Advisory Committee voted 16–0 in favour.[8] On December 5, 2017, semaglutide was approved by the US FDA. It can be used as both injection-type or oral-type drug.[9] The marketing authorization in EU was granted on 8 February 2018.[10] It is still under review by regulatory authorities in Japan, pending approval.[11]
Structure
In humans semaglutide is chemically similar to glucagon-like peptide-1 (GLP-1). The only differences are two amino acid substitutions at positions 8 and 34, where 2-aminoisobutyric acid and arginine are present, respectively.[12] In addition, lysine at position 26 is in its derivative form (acylated with stearic diacid).[13]
Mechanism of action
Semaglutide is a GLP-1 analog and functions as a GLP-1 agonist.[14]
References
- ↑ "Semaglutide Approval Status". drugs.com.
- ↑ "Novo Nordisk Files for Regulatory Approval of Once-Weekly Semaglutide with the FDA for the Treatment of Type 2 Diabetes" (Press release). Novo Nordisk. December 5, 2016.
- ↑ "Abstracts of the 48th EASD Annual Meeting of the European Association for the Study of Diabetes". Diabetologia. 55 (S1): 1–538. 2012. doi:10.1007/s00125-012-2688-9. PMID 22918257.
- ↑ Kalra, S; Gupta, Y (2015). "Once-weekly glucagon-like peptide 1 receptor agonists". The Journal of the Pakistan Medical Association. 65 (7): 796–798. PMID 26160096.
- ↑ "Efficacy and Safety of Semaglutide Versus Dulaglutide as add-on to Metformin in Subjects With Type 2 Diabetes". clinicaltrials.gov. U.S. National Library of Medicine. 25 September 2017. Retrieved 24 October 2017.
- ↑ Blundell, John; Finlayson, Graham; Axelsen, Mads; Flint, Anne; Gibbons, Catherine; Kvist, Trine; Hjerpsted, Julie B. (2017). "Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity". Diabetes, Obesity and Metabolism. 19 (9): 1242–1251. doi:10.1111/dom.12932.
- ↑ "Drug can dramatically reduce weight of people with obesity". ScienceDaily. 23 October 2017. Retrieved 24 October 2017.
- ↑ "Development Status and FDA Approval Process for semaglutide". Drugs.com. 2017. Retrieved 24 October 2017.
- ↑ Davies, Melanie; Pieber, Thomas R.; Hartoft-Nielsen, Marie-Louise; Hansen, Oluf K. H.; Jabbour, Serge; Rosenstock, Julio (2017). "Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes". JAMA. 318 (15): 1460–1470. doi:10.1001/jama.2017.14752.
- ↑ "Novo Nordisk A/S: Ozempic® (semaglutide) approved in the EU for the treatment of type 2 diabetes". globenewswire.com. 9 February 2018. Retrieved 2018-08-19.
- ↑ Ozempic (semaglutide) approved in the US, https://www.novonordisk.com/media/news-details.2154210.html
- ↑ Lau, Jesper; Bloch, Paw; Schäffer, Lauge; Pettersson, Ingrid; Spetzler, Jane; Kofoed, Jacob; Madsen, Kjeld; Knudsen, Lotte Bjerre; et al. (2015). "Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide". Journal of Medicinal Chemistry. 58 (18): 7370–7380. doi:10.1021/acs.jmedchem.5b00726. PMID 26308095.
- ↑ Gotfredsen, C. F.; Molck, A.-M.; Thorup, I.; Nyborg, N. C. B.; Salanti, Z.; Knudsen, L. B.; Larsen, M. O. (2014). "The Human GLP-1 Analogs Liraglutide and Semaglutide: Absence of Histopathological Effects on the Pancreas in Nonhuman Primates" (PDF). Diabetes. 63 (7): 2486–2497. doi:10.2337/db13-1087. PMID 24608440.
- ↑ Marso, Steven P.; Bain, Stephen C.; Consoli, Agostino; Eliaschewitz, Freddy G.; Jódar, Esteban; Leiter, Lawrence A.; Lingvay, Ildiko; Rosenstock, Julio; Seufert, Jochen; Warren, Mark L.; Woo, Vincent; Hansen, Oluf; Holst, Anders G.; Pettersson, Jonas; Vilsbøll, Tina (2016). "Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes". New England Journal of Medicine. 375 (19): 1834. doi:10.1056/NEJMoa1607141. PMID 27633186.