Insulin aspart
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605013 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C256H381N65O79S6 |
| Molar mass | 5825.8 g/mol |
| | |
Insulin aspart is a fast-acting insulin analog marketed by Novo Nordisk as NovoLog/NovoRapid. It is a manufactured form of human insulin; where a single amino acid has been exchanged. This change helps the fast-acting insulin analog be absorbed quickly into the bloodstream. As a result, it starts working in minutes, which allows one to take insulin and eat right away. Fast-acting insulin analogs are considered to act similarly to the way insulin is released in people without diabetes mellitus.
Novolog allows for a flexible dosing schedule, which allows patients to adjust their insulin according to any changes in their eating habits.[1]
The safety and efficacy of insulin aspart (NovoLog/NovoRapid) in real-life clinical practice was evaluated in the A1chieve study.
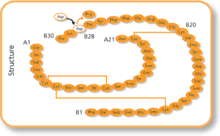
It was created through recombinant DNA technology so that the amino acid, B28, which is normally proline, is substituted with an aspartic acid residue. This analog has increased charge repulsion, which prevents the formation of hexamers, to create a faster-acting insulin. The sequence was inserted into the yeast genome, and the yeast expressed the insulin analog, which was then harvested from a bioreactor.
According to JDRF, insulin aspart was approved for marketing in the United States by the Food and Drug Administration in June 2000.
Chemical properties
The components of insulin aspart are as follows:
- Metal ion – zinc (19.6 μg/mL)
- Buffer – disodium hydrogen phosphate dihydrate (1.25 mg/mL)
- Preservatives – m-cresol (1.72 mg/mL) and phenol (1.50 mg/mL)
- Isotonicity agents – glycerin (16 mg/mL) and sodium chloride (0.58 mg/mL).
Action time
The onset of action is approximately 15 minutes, the peak action is reached in 45–90 minutes, and the duration is 3–5 hours. However, as with all insulin, these numbers are based on averages, and vary between individuals due to blood flow, injection site, temperature and exercise.[2][3]
Temperature
It has been debated whether or not insulin aspart (or NovoLog/NovoRapid) should be kept refrigerated at all times. The manufacturer claims it can last 28 days without refrigeration, as long as it is kept below 86 °F / 30 °C.[4] Above these temperatures, the potency of the insulin quickly degrades, rendering it less effective.
Usage
Insulin aspart can be used in CSII pumps and Flexpen, Novopen delivery devices for subcutaneous injection. Additionally, it can be used with an injection port such as the I-port.[5]
Novolog® is used for the treatment of patients with diabetes mellitus to control hyperglycemia.[6] Novolog® has a more rapid onset, and a shorter duration of activity than normal human insulin. Therefore, Novolog® given by injection should normally be used in a regimen with long-acting or intermediate insulin.[6] Novolog® can also be used with external insulin pumps. The insulin in reservoirs of insulin pumps and infusion sets should be changed every 48 hours to avoid insulin degradation and loss of preservative.[3] The following insulin pumps have been used in Novolog® clinical or in vitro studies conducted by Novo Nordisk: Medtronic Paradigm® 512 and 712, Minimed 508, Disetronic® D-TRON® and H-TRON®.[7]
Variations on Insulin Aspart
NovoLog Mix 70/30 is a product marketed by Novo Nordisk which contains 30% insulin aspart and 70% insulin aspart protamine. The insulin aspart protamine portion is a crystalline form of insulin aspart, which delays the action of the insulin, giving it a prolonged absorption profile after injection. The combination of the fast-acting form and the long-acting form allows the patient to receive fewer injections over the course of the day.[8]
The components of NovoLog Mix 70/30 are as follows:
- Metal ion – zinc (19.6 μg/mL)
- Buffer – dibasic sodium phosphate (1.25 mg/mL)
- Preservatives – m-cresol (1.72 mg/mL) and phenol (1.50 mg/mL)
- Isotonicity agents – sodium chloride (0.58 mg/mL) and mannitol (36.4 mg/mL)
- Modifying protein – protamine (0.33 mg/mL)
The pH is 7.2–7.44.[2]
NovoLog Mix is marketed to be used with the Novo Nordisk FlexPen.[9] The onset of action is less than 30 minutes, the peak action is reached in 1–4 hours, and the duration is less than 24 hours.[2]
NovoLog Mix is marketed in some countries as NovoMix 30.[10]
NovoLog® is available in different forms. There are 10-mL vials, 3-mL PenFill® cartridges, and 3-mL NovoLog® FlexPens®. The NovoLog® FlexPen® is a way to take NovoLog® via a prefilled, disposable insulin pen delivery system. The NovoLog® FlexPen® can be used for up to 28 days without being refrigerated once it has been used. The large, clear dosing window allows for accurate dose setting and adjustment. NovoLog® is also supplied in 3-mL PenFill® cartridges and can be used in the NovoPen® 3 insulin delivery system.[1]
Efficacy
There is a lack of compelling evidence to conclude superiority of insulin aspart over human insulin with regard to efficacy[11] leading some to good question the shifting of patients from human insulin to this modern insulin.[12]
Side effects
The safety of Novolog in patients with diabetes has been evaluated in several clinical studies. Novolog was compared with regular human insulin, and there was no difference in the frequency of adverse effects between the two treatments. The side effects that are commonly associated with insulin therapy include: allergic reactions, injection site irritation, rashes, and hypoglycemia.[1] The most common side effect is hypoglycemia. Long-term use of insulin, including Novolog®, can cause lipodystrophy at the site of repeated injections or infusion. To reduce the risk of lipodystrophy, rotate the injection sites within the same region. Weight gain can also occur with the use of Novolog® and it has been attributed to anabolic effects of insulin and a decrease in glucosuria. Use of Novolog® has also been associated with sodium retention and edema.[7]
External links
References
- 1 2 3 "Novolog: most FAQ". Retrieved 2011. Check date values in:
|accessdate=(help) - 1 2 3 4 Crommelin DJA, Sindelar RD, Meibohm B. 2008. Pharmaceutical Biotechnology: Fundamentals and Applications. New York, NY: Informa Healthcare USA, Inc. p 270.
- 1 2 FDA. NovoLog Insulin Aspart (rDNA origin) Injection. 7 June 2000. http://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20986lbl.pdf
- ↑ "NovoLog® Storage". Novolog.com. Retrieved 2016-04-23.
- ↑ "Aspart insulin (rDNA origin) injection". Archived from the original on 2007-06-10. Retrieved 2007-06-08.
- 1 2 "Novolog: insulin aspart" (PDF).
- 1 2 "Novolog: insulin aspart (rDNA origin) injection" (PDF). Retrieved October 2010. Check date values in:
|accessdate=(help) - ↑ Rx List: NovoLog Mix 70/30. 6 August 2008. http://www.rxlist.com/novolog-mix-70-30-drug.htm
- ↑ Novo Nordisk: NovoLog Mix 70/30. 2008. http://www.novologmix70-30.com/starting-with-novolog-mix.asp
- ↑ "NovoMix 30 Penfill 100 units/ml, NovoMix 30 FlexPen 100 units/ml - Summary of Product Characteristics (SmPC) - (eMC)". www.medicines.org.uk.
- ↑ Insulin aspart for diabetes mellitus http://www.ti.ubc.ca/fr/insulin-aspart-diabetes-mellitus-last-update-march-2007
- ↑ Cohen, D; Carter, P (2010). "How small changes led to big profits for insulin manufacturers". BMJ. 341: c7139. doi:10.1136/bmj.c7139. PMID 21159773.