Tyrannosaurus
Tyrannosaurus[nb 1] is a genus of coelurosaurian theropod dinosaur. The species Tyrannosaurus rex (rex meaning "king" in Latin), often called T. rex or colloquially T-Rex, is one of the most well-represented of the large theropods. Tyrannosaurus lived throughout what is now western North America, on what was then an island continent known as Laramidia. Tyrannosaurus had a much wider range than other tyrannosaurids. Fossils are found in a variety of rock formations dating to the Maastrichtian age of the upper Cretaceous Period, 68 to 66 million years ago. It was the last known member of the tyrannosaurids, and among the last non-avian dinosaurs to exist before the Cretaceous–Paleogene extinction event.
| Tyrannosaurus | |
|---|---|
 | |
| Reconstruction of the T. rex type specimen (CM 9380) at the Carnegie Museum of Natural History | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Dinosauria |
| Clade: | Saurischia |
| Clade: | Theropoda |
| Family: | †Tyrannosauridae |
| Subfamily: | †Tyrannosaurinae |
| Genus: | †Tyrannosaurus Osborn, 1905 |
| Type species | |
| †Tyrannosaurus rex Osborn, 1905 | |
| Other species | |
| |
| Synonyms | |
|
Genus synonymy
Species synonymy
| |
Like other tyrannosaurids, Tyrannosaurus was a bipedal carnivore with a massive skull balanced by a long, heavy tail. Relative to its large and powerful hind limbs, Tyrannosaurus forelimbs were short but unusually powerful for their size and had two clawed digits. The most complete specimen measures up to 12.3 m (40 ft) in length though T. rex could grow to lengths of over 12.3 m (40 ft), up to 3.66 meters (12 ft) tall at the hips, and according to most modern estimates 8.4 metric tons (9.3 short tons) to 14 metric tons (15.4 short tons) in weight. Although other theropods rivaled or exceeded Tyrannosaurus rex in size, it is still among the largest known land predators and is estimated to have exerted the strongest bite force among all terrestrial animals. By far the largest carnivore in its environment, Tyrannosaurus rex was most likely an apex predator, preying upon hadrosaurs, armored herbivores like ceratopsians and ankylosaurs, and possibly sauropods. Some experts have suggested the dinosaur was primarily a scavenger. The question of whether Tyrannosaurus was an apex predator or a pure scavenger was among the longest debates in paleontology. Most paleontologists today accept that Tyrannosaurus was both an active predator and a scavenger.
More than fifty major specimens of Tyrannosaurus rex have been identified, some of which are nearly complete skeletons. Soft tissue and proteins have been reported in at least one of these specimens. The abundance of fossil material has allowed significant research into many aspects of its biology, including its life history and biomechanics. The feeding habits, physiology and potential speed of Tyrannosaurus rex are a few subjects of debate. Its taxonomy is also controversial, as some scientists consider Tarbosaurus bataar from Asia to be a second Tyrannosaurus species while others maintain Tarbosaurus is a separate genus. Several other genera of North American tyrannosaurids have also been synonymized with Tyrannosaurus.
As the archetypal theropod, Tyrannosaurus has been one of the best-known dinosaurs since the early 20th century, and has been featured in film, advertising, postal stamps, and many other media.
Description
Size
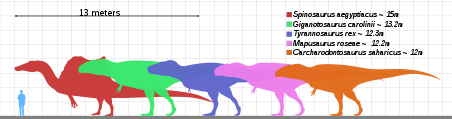
Tyrannosaurus rex was one of the largest land carnivores of all time. One of the largest and the most complete specimens, nicknamed Sue (FMNH PR2081), is located at the Field Museum of Natural History. Sue measured 12.3–12.8 meters (40–42 ft) long,[2][3] was 3.66 meters (12 ft) tall at the hips,[4] and according to the most recent studies, using a variety of techniques, estimated to have weighed between 8.4 metric tons (9.3 short tons) to 14 metric tons (15.4 short tons).[3][5] A specimen nicknamed Scotty (RSM P2523.8), located at the Royal Saskatchewan Museum, is reported to measure 13 m (43 ft) in length. Using a mass estimation technique that extrapolates from the circumference of the femur, Scotty was estimated as the largest known specimen at 8.8 metric tons (9.7 short tons) in weight.[6][7]
Not every adult Tyrannosaurus specimen recovered is as big. Historically average adult mass estimates have varied widely over the years, from as low as 4.5 metric tons (5.0 short tons),[8][9] to more than 7.2 metric tons (7.9 short tons),[10] with most modern estimates ranging between 5.4 metric tons (6.0 short tons) and 8.0 metric tons (8.8 short tons).[3][11][12][13][14]
Skeleton
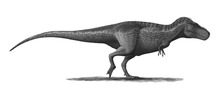
The largest known Tyrannosaurus rex skull is 1.52 meters (5 ft) in length.[4] Large fenestrae (openings) in the skull reduced weight, as in all carnivorous theropods. In other respects Tyrannosaurus's skull was significantly different from those of large non-tyrannosaurid theropods. It was extremely wide at the rear but had a narrow snout, allowing unusually good binocular vision.[15][16] The skull bones were massive and the nasals and some other bones were fused, preventing movement between them; but many were pneumatized (contained a "honeycomb" of tiny air spaces) and thus lighter. These and other skull-strengthening features are part of the tyrannosaurid trend towards an increasingly powerful bite, which easily surpassed that of all non-tyrannosaurids.[17][18][19] The tip of the upper jaw was U-shaped (most non-tyrannosauroid carnivores had V-shaped upper jaws), which increased the amount of tissue and bone a tyrannosaur could rip out with one bite, although it also increased the stresses on the front teeth.[20]
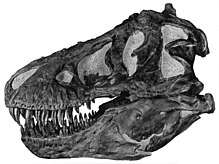
The teeth of Tyrannosaurus rex displayed marked heterodonty (differences in shape).[21][22] The premaxillary teeth, four per side at the front of the upper jaw, were closely packed, D-shaped in cross-section, had reinforcing ridges on the rear surface, were incisiform (their tips were chisel-like blades) and curved backwards. The D-shaped cross-section, reinforcing ridges and backwards curve reduced the risk that the teeth would snap when Tyrannosaurus bit and pulled. The remaining teeth were robust, like "lethal bananas" rather than daggers, more widely spaced and also had reinforcing ridges.[23] Those in the upper jaw, twelve per side in mature individuals,[21] were larger than their counterparts of the lower jaw, except at the rear. The largest found so far is estimated to have been 30.5 centimeters (12 in) long including the root when the animal was alive, making it the largest tooth of any carnivorous dinosaur yet found.[24] The lower jaw was robust. Its front dentary bone bore thirteen teeth. Behind the tooth row, the lower jaw became notably taller.[21]
The vertebral column of Tyrannosaurus consisted of ten neck vertebrae, thirteen back vertebrae and five sacral vertebrae. The number of tail vertebrae is unknown and could well have varied between individuals but probably numbered at least forty. Sue was mounted with forty-seven of such caudal vertebrae.[21] The neck of Tyrannosaurus rex formed a natural S-shaped curve like that of other theropods. Compared to these, it was exceptionally short, deep and muscular to support the massive head. The second vertebra, the axis, was especially short. The remaining neck vertebrae were weakly opisthocoelous, i.e. with a convex front of the vertebral body and a concave rear. The vertebral bodies had single pleurocoels, pneumatic depressions created by air sacs, on their sides.[21] The vertebral bodies of the torso were robust but with a narrow waist. Their undersides were keeled. The front sides were concave with a deep vertical trough. They had large pleurocoels. Their neural spines had very rough front and rear sides for the attachment of strong tendons. The sacral vertebrae were fused to each other, both in their vertebral bodies and neural spines. They were pneumatized. They were connected to the pelvis by transverse processes and sacral ribs. The tail was heavy and moderately long, in order to balance the massive head and torso and to provide space for massive locomotor muscles that attached to the thighbones. The thirteenth tail vertebra formed the transition point between the deep tail base and the middle tail that was stiffened by rather long front articulation processes. The underside of the trunk was covered by eighteen or nineteen pairs of segmented belly ribs.[21]
.jpg)
The shoulder girdle was longer than the entire forelimb. The shoulder blade had a narrow shaft but was exceptionally expanded at its upper end. It connected via a long forward protrusion to the coracoid, which was rounded. Both shoulder blades were connected by a small furcula. The paired breast bones possibly were made of cartilage only.[21]
The forelimb or arm was very short. The upper arm bone, the humerus, was short but robust. It had a narrow upper end with an exceptionally rounded head. The lower arm bones, the ulna and radius, were straight elements, much shorter than the humerus. The second metacarpal was longer and wider than the first, whereas normally in theropods the opposite is true. The forelimbs had only two clawed fingers,[21] along with an additional splint-like small third metacarpal representing the remnant of a third digit.[25]
The pelvis was a large structure. Its upper bone, the ilium, was both very long and high, providing an extensive attachment area for hindlimb muscles. The front pubic bone ended in an enormous pubic boot, longer than the entire shaft of the element. The rear ischium was slender and straight, pointing obliquely to behind and below.[21]
In contrast to the arms, the hindlimbs were among the longest in proportion to body size of any theropod. In the foot, the metatarsus was "arctometatarsalian", meaning that the part of the third metatarsal near the ankle was pinched. The third metatarsal was also exceptionally sinuous.[21] Compensating for the immense bulk of the animal, many bones throughout the skeleton were hollowed, reducing its weight without significant loss of strength.[21]
Skin and possible feathers
The discovery of feathered dinosaurs led to debates if, and to what extent, Tyrannosaurus might have been feathered.[26][27] Filamentous structures, which are commonly recognized as the precursors of feathers, have been reported in the small-bodied, basal tyrannosauroid Dilong paradoxus from the Early Cretaceous Yixian Formation of China in 2004.[28] Because integumentary impressions of larger tyrannosauroids known at that time showed evidence of scales, the researchers who studied Dilong speculated that insulating feathers might have been lost by larger species due to their smaller surface-to-volume ratio.[28] The subsequent discovery of the giant species Yutyrannus huali, also from the Yixian, showed that even some large tyrannosauroids had feathers covering much of their bodies, casting doubt on the hypothesis that they were a size-related feature.[29] A 2017 study reviewed known skin impressions of tyrannosaurids, including those of a Tyrannosaurus specimen nicknamed "Wyrex" (BHI 6230) which preserves patches of mosaic scales on the tail, hip, and neck.[30] The study concluded that feather covering of large tyrannosaurids such as Tyrannosaurus was, if present, limited to the upper side of the trunk.[26]
A conference abstract published in 2016 posited that theropods such as Tyrannosaurus had their upper teeth covered in lips, instead of bare teeth as seen in crocodilians. This was based on the presence of enamel, which according to the study needs to remain hydrated, an issue not faced by aquatic animals like crocodilians.[31] A 2017 analytical study proposed that tyrannosaurids had large, flat scales on their snouts instead of lips.[32][33]
History of research
Earliest finds
Teeth from what is now documented as a Tyrannosaurus rex were found in 1874 by Arthur Lakes near Golden, Colorado. In the early 1890s, John Bell Hatcher collected postcranial elements in eastern Wyoming. The fossils were believed to be from the large species Ornithomimus grandis (now Deinodon) but are now considered Tyrannosaurus rex remains.[34]
In 1892, Edward Drinker Cope found two vertebral fragments of large dinosaur. Cope believed the fragments belonged to an "agathaumid" (ceratopsid) dinosaur, and named them Manospondylus gigas, meaning "giant porous vertebra", in reference to the numerous openings for blood vessels he found in the bone.[34] The M. gigas remains were, in 1907, identified by Hatcher as those of a theropod rather than a ceratopsid.[35] Henry Fairfield Osborn recognized the similarity between Manospondylus gigas and Tyrannosaurus rex as early as 1917, by which time the second vertebra had been lost. Owing to the fragmentary nature of the Manospondylus vertebrae, Osborn did not synonymize the two genera, instead considering the older genus indeterminate.[36] In June 2000, the Black Hills Institute found around 10% of a Tyrannosaurus skeleton (BHI 6248) at a site that might have been the original M. gigas locality.[30]
Skeleton discovery and naming
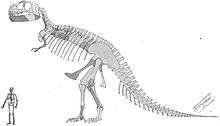
Barnum Brown, assistant curator of the American Museum of Natural History, found the first partial skeleton of Tyrannosaurus rex in eastern Wyoming in 1900. Brown found another partial skeleton in the Hell Creek Formation in Montana in 1902, comprising approximately 34 fossilized bones.[37] Writing at the time Brown said "Quarry No. 1 contains the femur, pubes, humerus, three vertebrae and two undetermined bones of a large Carnivorous Dinosaur not described by Marsh.... I have never seen anything like it from the Cretaceous".[38] Henry Fairfield Osborn, president of the American Museum of Natural History, named the second skeleton Tyrannosaurus rex in 1905. The generic name is derived from the Greek words τύραννος (tyrannos, meaning "tyrant") and σαῦρος (sauros, meaning "lizard"). Osborn used the Latin word rex, meaning "king", for the specific name. The full binomial therefore translates to "tyrant lizard the king" or "King Tyrant Lizard", emphasizing the animal's size and perceived dominance over other species of the time. Osborn named the other specimen Dynamosaurus imperiosus in a paper in 1905.[37] In 1906, Osborn recognized that the two skeletons were from the same species and selected Tyrannosaurus as the preferred name.[39]

The original Dynamosaurus material resides in the collections of the Natural History Museum, London.[40] In 1941, the T. rex type specimen was sold to the Carnegie Museum of Natural History in Pittsburgh, Pennsylvania, for $7,000.[38] Dynamosaurus would later be honored by the 2018 description of another species of tyrannosaurid by Andrew McDonald and colleagues, Dynamoterror dynastes, whose name was chosen in reference to the 1905 name, as it had been a "childhood favorite" of McDonald's.[41]
From the 1910s through the end of the 1950s, Barnum's discoveries remained the only specimens of Tyrannosaurus, as the Great Depression and wars kept many paleontologists out of the field.[30]
Resurgent interest


Beginning in the 1960s, there was renewed interest in Tyrannosaurus, resulting in recovery of 42 skeletons (5-80% complete by bone count) from Western North America.[30] In 1967, Dr. William MacMannis located and recovered the skeleton named "MOR 008", which is 15% complete by bone count and has a reconstructed skull displayed at the Museum of the Rockies. The 1990s saw numerous discoveries, with nearly twice as many finds as in all previous years, including two of the most complete skeletons found to date: Sue and Stan.[30]
Sue Hendrickson, an amateur paleontologist, discovered the most complete (approximately 85%) and largest Tyrannosaurus skeleton in the Hell Creek Formation on August 12, 1990. The specimen Sue, named after the discoverer, was the object of a legal battle over its ownership. In 1997, the litigation was settled in favor of Maurice Williams, the original land owner. The fossil collection was purchased by the Field Museum of Natural History at auction for $7.6 million, making it the most expensive dinosaur skeleton to date. From 1998 to 1999, Field Museum of Natural History staff spent over 25,000 hours taking the rock off the bones.[42] The bones were then shipped to New Jersey where the mount was constructed, then shipped back to Chicago for the final assembly. The mounted skeleton opened to the public on May 17, 2000 in the Field Museum of Natural History. A study of this specimen's fossilized bones showed that Sue reached full size at age 19 and died at the age of 28, the longest estimated life of any tyrannosaur known.[43]

Another Tyrannosaurus, nicknamed Stan (BHI 3033), in honor of amateur paleontologist Stan Sacrison, was recovered from the Hell Creek Formation in 1992. Stan is the second most complete skeleton found, with 199 bones recovered representing 70% of the total.[44] This tyrannosaur also had many bone pathologies, including broken and healed ribs, a broken (and healed) neck, and a substantial hole in the back of its head, about the size of a Tyrannosaurus tooth.[45]
In 1998, Bucky Derflinger noticed one of Bucky's toes exposed above ground, making Derflinger, who was 20 years old at the time, the youngest person to discover a Tyrannosaurus. The specimen was a young adult, 3.0 metres (10 ft) tall and 11 metres (35 ft) long. Bucky is the first Tyrannosaurus to be found that preserved a furcula (wishbone). Bucky is permanently displayed at The Children's Museum of Indianapolis.[46]

In the summer of 2000, crews organized by Jack Horner discovered five Tyrannosaurus skeletons near the Fort Peck Reservoir.[47] In 2001, a 50% complete skeleton of a juvenile Tyrannosaurus was discovered in the Hell Creek Formation by a crew from the Burpee Museum of Natural History. Dubbed Jane (BMRP 2002.4.1), the find was thought to be the first known skeleton of a pygmy tyrannosaurid, Nanotyrannus, but subsequent research revealed that it is more likely a juvenile Tyrannosaurus, and the most complete juvenile example known;[48] Jane is exhibited at the Burpee Museum of Natural History.[49] In 2002, a skeleton named Wyrex, discovered by amateur collectors Dan Wells and Don Wyrick, had 114 bones and was 38% complete. The dig was concluded over 3 weeks in 2004 by the Black Hills Institute with the first live online Tyrannosaurus excavation providing daily reports, photos, and video.[30]
In 2006, Montana State University revealed that it possessed the largest Tyrannosaurus skull yet discovered (from a specimen named MOR 008), measuring 5 feet (152 cm) long.[50] Subsequent comparisons indicated that the longest head was 136.5 centimetres (53.7 in) (from specimen LACM 23844) and the widest head was 90.2 centimetres (35.5 in) (from Sue).[51]
Footprints

Two isolated fossilized footprints have been tentatively assigned to Tyrannosaurus rex. The first was discovered at Philmont Scout Ranch, New Mexico, in 1983 by American geologist Charles Pillmore. Originally thought to belong to a hadrosaurid, examination of the footprint revealed a large 'heel' unknown in ornithopod dinosaur tracks, and traces of what may have been a hallux, the dewclaw-like fourth digit of the tyrannosaur foot. The footprint was published as the ichnogenus Tyrannosauripus pillmorei in 1994, by Martin Lockley and Adrian Hunt. Lockley and Hunt suggested that it was very likely the track was made by a Tyrannosaurus rex, which would make it the first known footprint from this species. The track was made in what was once a vegetated wetland mud flat. It measures 83 centimeters (33 in) long by 71 centimeters (28 in) wide.[52]
A second footprint that may have been made by a Tyrannosaurus was first reported in 2007 by British paleontologist Phil Manning, from the Hell Creek Formation of Montana. This second track measures 72 centimeters (28 in) long, shorter than the track described by Lockley and Hunt. Whether or not the track was made by Tyrannosaurus is unclear, though Tyrannosaurus and Nanotyrannus are the only large theropods known to have existed in the Hell Creek Formation.[53][54]
A set of footprints in Glenrock, Wyoming dating to the Maastrichtian stage of the Late Cretaceous and hailing from the Lance Formation were described by Scott Persons, Phil Currie et al. in January 2016, and are believed to belong to either a juvenile Tyrannosaurus rex or the dubious tyrannosaurid Nanotyrannus lancensis. From measurements and based on the positions of the footprints, the animal was believed to be traveling at a walking speed of around 2.8 to 5 miles per hour and was estimated to have a hip height of 1.56 m (5.1 ft) to 2.06 m (6.8 ft).[55][56][57] A follow-up paper appeared in 2017, increasing the speed estimations by 50-80%.[58]
Classification
Tyrannosaurus is the type genus of the superfamily Tyrannosauroidea, the family Tyrannosauridae, and the subfamily Tyrannosaurinae; in other words it is the standard by which paleontologists decide whether to include other species in the same group. Other members of the tyrannosaurine subfamily include the North American Daspletosaurus and the Asian Tarbosaurus,[48][59] both of which have occasionally been synonymized with Tyrannosaurus.[60] Tyrannosaurids were once commonly thought to be descendants of earlier large theropods such as megalosaurs and carnosaurs, although more recently they were reclassified with the generally smaller coelurosaurs.[20]

In 1955, Soviet paleontologist Evgeny Maleev named a new species, Tyrannosaurus bataar, from Mongolia.[61] By 1965, this species had been renamed Tarbosaurus bataar.[62] Despite the renaming, many phylogenetic analyses have found Tarbosaurus bataar to be the sister taxon of Tyrannosaurus rex,[59] and it has often been considered an Asian species of Tyrannosaurus.[20][63][64] A recent redescription of the skull of Tarbosaurus bataar has shown that it was much narrower than that of Tyrannosaurus rex and that during a bite, the distribution of stress in the skull would have been very different, closer to that of Alioramus, another Asian tyrannosaur.[65] A related cladistic analysis found that Alioramus, not Tyrannosaurus, was the sister taxon of Tarbosaurus, which, if true, would suggest that Tarbosaurus and Tyrannosaurus should remain separate.[48] The discovery and description of Qianzhousaurus in 2014, would disprove this and reveal that Alioramus belonged to the clade Alioramini.[66][67] The discovery of the tyrannosaurid Lythronax further indicates that Tarbosaurus and Tyrannosaurus are closely related, forming a clade with fellow Asian tyrannosaurid Zhuchengtyrannus, with Lythronax being their sister taxon.[68][69] A further study from 2016 by Steve Brusatte, Thomas Carr et al., also indicates Tyrannosaurus may have been an immigrant from Asia, as well as a possible descendant of Tarbosaurus. The study further indicates the possibility that Tyrannosaurus may have driven other tyrannosaurids that were native to North America extinct through competition.[70] Other finds in 2006 indicate giant tyrannosaurs may have been present in North America as early as 75 million years ago. Whether or not this specimen belongs to Tyrannosaurus rex, a new species of Tyrannosaurus, or a new genus entirely is still unknown.[71]
Other tyrannosaurid fossils found in the same formations as Tyrannosaurus rex were originally classified as separate taxa, including Aublysodon and Albertosaurus megagracilis,[60] the latter being named Dinotyrannus megagracilis in 1995.[72] These fossils are now universally considered to belong to juvenile Tyrannosaurus rex.[73] A small but nearly complete skull from Montana, 60 centimeters (2.0 ft) long, may be an exception. This skull was originally classified as a species of Gorgosaurus (G. lancensis) by Charles W. Gilmore in 1946,[74] but was later referred to a new genus, Nanotyrannus.[75] Opinions remain divided on the validity of N. lancensis. Many paleontologists consider the skull to belong to a juvenile Tyrannosaurus rex.[76] There are minor differences between the two species, including the higher number of teeth in N. lancensis, which lead some scientists to recommend keeping the two genera separate until further research or discoveries clarify the situation.[59][77]
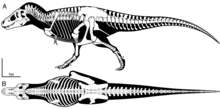

In 2001, various tyrannosaurid teeth and a metatarsal unearthed in a quarry near Zhucheng, China were assigned by Chinese paleontologist Hu Chengzhi to the newly erected Tyrannosaurus zhuchengensis. However, in a nearby site, a right maxilla and left jawbone were assigned to the newly erected tyrannosaurid genus Zhuchengtyrannus in 2011, and it is possible T. zhuchengensis is synonymous with Zhuchengtyrannus. In any case, T. zhuchengensis is considered to be a nomen dubium as the holotype lacks diagnostic features below the level Tyrannosaurinae.[78]
Below is the cladogram of Tyrannosauridae based on the phylogenetic analysis conducted by Loewen et al. in 2013.[68]
| Tyrannosauridae |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Paleobiology
Life history

The identification of several specimens as juvenile Tyrannosaurus rex has allowed scientists to document ontogenetic changes in the species, estimate the lifespan, and determine how quickly the animals would have grown. The smallest known individual (LACM 28471, the "Jordan theropod") is estimated to have weighed only 30 kg (66 lb), while the largest, such as FMNH PR2081 (Sue) most likely weighed about 5,650 kg (12,460 lb). Histologic analysis of Tyrannosaurus rex bones showed LACM 28471 had aged only 2 years when it died, while Sue was 28 years old, an age which may have been close to the maximum for the species.[11]
Histology has also allowed the age of other specimens to be determined. Growth curves can be developed when the ages of different specimens are plotted on a graph along with their mass. A Tyrannosaurus rex growth curve is S-shaped, with juveniles remaining under 1,800 kg (4,000 lb) until approximately 14 years of age, when body size began to increase dramatically. During this rapid growth phase, a young Tyrannosaurus rex would gain an average of 600 kg (1,300 lb) a year for the next four years. At 18 years of age, the curve plateaus again, indicating that growth slowed dramatically. For example, only 600 kg (1,300 lb) separated the 28-year-old Sue from a 22-year-old Canadian specimen (RTMP 81.12.1).[11] A 2004 histological study performed by different workers corroborates these results, finding that rapid growth began to slow at around 16 years of age.[79]

A study by Hutchinson et al. in 2011 corroborated the previous estimation methods in general, but their estimation of peak growth rates is significantly higher; it found that the "maximum growth rates for T. rex during the exponential stage are 1790 kg/year".[3] Although these results were much higher than previous estimations, the authors noted that these results significantly lowered the great difference between its actual growth rate and the one which would be expected of an animal of its size.[3] The sudden change in growth rate at the end of the growth spurt may indicate physical maturity, a hypothesis which is supported by the discovery of medullary tissue in the femur of a 16 to 20-year-old Tyrannosaurus rex from Montana (MOR 1125, also known as B-rex). Medullary tissue is found only in female birds during ovulation, indicating that B-rex was of reproductive age.[80] Further study indicates an age of 18 for this specimen.[81] In 2016, it was finally confirmed by Mary Higby Schweitzer and Lindsay Zanno et al that the soft tissue within the femur of MOR 1125 was medullary tissue. This also confirmed the identity of the specimen as a female. The discovery of medullary bone tissue within Tyrannosaurus may prove valuable in determining the sex of other dinosaur species in future examinations, as the chemical makeup of medullary tissue is unmistakable.[82] Other tyrannosaurids exhibit extremely similar growth curves, although with lower growth rates corresponding to their lower adult sizes.[83]
An additional study published on January 1, 2020 by Woodward et al, for the journal Science Advances indicates that during their growth from juvenile to adult, Tyrannosaurus was capable of slowing down its growth to counter environmental factors such as lack of food. The study, focusing on two juvenile specimens between 13 and 15 years old housed at the Burpee Museum in Illinois, indicates that the rate of maturation for Tyrannosaurus was dependent on resource abundance. This study also indicates that in such changing environments, Tyrannosaurus was particularly well-suited to an environment that shifted yearly in regards to resource abundance, hinting that other midsize predators might have had difficulty surviving in such harsh conditions and explaining the niche partitioning between juvenile and adult tyrannosaurs. The study further indicates that Tyrannosaurus and the dubious genus Nanotyrannus are synonymous, due to analysis of the growth rings in the bones of the two specimens studied.[84][85]
Over half of the known Tyrannosaurus rex specimens appear to have died within six years of reaching sexual maturity, a pattern which is also seen in other tyrannosaurs and in some large, long-lived birds and mammals today. These species are characterized by high infant mortality rates, followed by relatively low mortality among juveniles. Mortality increases again following sexual maturity, partly due to the stresses of reproduction. One study suggests that the rarity of juvenile Tyrannosaurus rex fossils is due in part to low juvenile mortality rates; the animals were not dying in large numbers at these ages, and so were not often fossilized. This rarity may also be due to the incompleteness of the fossil record or to the bias of fossil collectors towards larger, more spectacular specimens.[83] In a 2013 lecture, Thomas Holtz Jr. suggested that dinosaurs "lived fast and died young" because they reproduced quickly whereas mammals have long life spans because they take longer to reproduce.[86] Gregory S. Paul also writes that Tyrannosaurus reproduced quickly and died young, but attributes their short life spans to the dangerous lives they lived.[87]
Sexual dimorphism

As the number of known specimens increased, scientists began to analyze the variation between individuals and discovered what appeared to be two distinct body types, or morphs, similar to some other theropod species. As one of these morphs was more solidly built, it was termed the 'robust' morph while the other was termed 'gracile'. Several morphological differences associated with the two morphs were used to analyze sexual dimorphism in Tyrannosaurus rex, with the 'robust' morph usually suggested to be female. For example, the pelvis of several 'robust' specimens seemed to be wider, perhaps to allow the passage of eggs.[88] It was also thought that the 'robust' morphology correlated with a reduced chevron on the first tail vertebra, also ostensibly to allow eggs to pass out of the reproductive tract, as had been erroneously reported for crocodiles.[89]
In recent years, evidence for sexual dimorphism has been weakened. A 2005 study reported that previous claims of sexual dimorphism in crocodile chevron anatomy were in error, casting doubt on the existence of similar dimorphism between Tyrannosaurus rex sexes.[90] A full-sized chevron was discovered on the first tail vertebra of Sue, an extremely robust individual, indicating that this feature could not be used to differentiate the two morphs anyway. As Tyrannosaurus rex specimens have been found from Saskatchewan to New Mexico, differences between individuals may be indicative of geographic variation rather than sexual dimorphism. The differences could also be age-related, with 'robust' individuals being older animals.[21]
Only a single Tyrannosaurus rex specimen has been conclusively shown to belong to a specific sex. Examination of B-rex demonstrated the preservation of soft tissue within several bones. Some of this tissue has been identified as a medullary tissue, a specialized tissue grown only in modern birds as a source of calcium for the production of eggshell during ovulation. As only female birds lay eggs, medullary tissue is only found naturally in females, although males are capable of producing it when injected with female reproductive hormones like estrogen. This strongly suggests that B-rex was female, and that she died during ovulation.[80] Recent research has shown that medullary tissue is never found in crocodiles, which are thought to be the closest living relatives of dinosaurs, aside from birds. The shared presence of medullary tissue in birds and theropod dinosaurs is further evidence of the close evolutionary relationship between the two.[91]
Posture

Like many bipedal dinosaurs, Tyrannosaurus rex was historically depicted as a 'living tripod', with the body at 45 degrees or less from the vertical and the tail dragging along the ground, similar to a kangaroo. This concept dates from Joseph Leidy's 1865 reconstruction of Hadrosaurus, the first to depict a dinosaur in a bipedal posture.[92] In 1915, convinced that the creature stood upright, Henry Fairfield Osborn, former president of the American Museum of Natural History, further reinforced the notion in unveiling the first complete Tyrannosaurus rex skeleton arranged this way. It stood in an upright pose for 77 years, until it was dismantled in 1992.[93]
By 1970, scientists realized this pose was incorrect and could not have been maintained by a living animal, as it would have resulted in the dislocation or weakening of several joints, including the hips and the articulation between the head and the spinal column.[94] The inaccurate AMNH mount inspired similar depictions in many films and paintings (such as Rudolph Zallinger's famous mural The Age of Reptiles in Yale University's Peabody Museum of Natural History)[95] until the 1990s, when films such as Jurassic Park introduced a more accurate posture to the general public.[96] Modern representations in museums, art, and film show Tyrannosaurus rex with its body approximately parallel to the ground with the tail extended behind the body to balance the head.[97]
Arms

When Tyrannosaurus rex was first discovered, the humerus was the only element of the forelimb known.[37] For the initial mounted skeleton as seen by the public in 1915, Osborn substituted longer, three-fingered forelimbs like those of Allosaurus.[36] A year earlier, Lawrence Lambe described the short, two-fingered forelimbs of the closely related Gorgosaurus.[98] This strongly suggested that Tyrannosaurus rex had similar forelimbs, but this hypothesis was not confirmed until the first complete Tyrannosaurus rex forelimbs were identified in 1989, belonging to MOR 555 (the "Wankel rex").[99] [100]The remains of Sue also include complete forelimbs.[21] Tyrannosaurus rex arms are very small relative to overall body size, measuring only 1 meter (3.3 ft) long, and some scholars have labelled them as vestigial. The bones show large areas for muscle attachment, indicating considerable strength. This was recognized as early as 1906 by Osborn, who speculated that the forelimbs may have been used to grasp a mate during copulation.[39] It has also been suggested that the forelimbs were used to assist the animal in rising from a prone position.[94]
Another possibility is that the forelimbs held struggling prey while it was killed by the tyrannosaur's enormous jaws. This hypothesis may be supported by biomechanical analysis. Tyrannosaurus rex forelimb bones exhibit extremely thick cortical bone, which has been interpreted as evidence that they were developed to withstand heavy loads. The biceps brachii muscle of an adult Tyrannosaurus rex was capable of lifting 199 kilograms (439 lb) by itself; other muscles such as the brachialis would work along with the biceps to make elbow flexion even more powerful. The M. biceps muscle of T. rex was 3.5 times as powerful as the human equivalent. A Tyrannosaurus rex forearm had a limited range of motion, with the shoulder and elbow joints allowing only 40 and 45 degrees of motion, respectively. In contrast, the same two joints in Deinonychus allow up to 88 and 130 degrees of motion, respectively, while a human arm can rotate 360 degrees at the shoulder and move through 165 degrees at the elbow. The heavy build of the arm bones, strength of the muscles, and limited range of motion may indicate a system evolved to hold fast despite the stresses of a struggling prey animal. In the first detailed scientific description of Tyrannosaurus forelimbs, paleontologists Kenneth Carpenter and Matt Smith dismissed notions that the forelimbs were useless or that Tyrannosaurus rex was an obligate scavenger.[101]
According to paleontologist Steven M. Stanley, the 1 metre (3.3 ft) arms of Tyrannosaurus rex were used for slashing prey, especially by using its claws to rapidly inflict long, deep gashes to its prey, although this concept is disputed by others believing the arms were used for grasping a sexual partner.[102]
Soft tissue
In the March 2005 issue of Science, Mary Higby Schweitzer of North Carolina State University and colleagues announced the recovery of soft tissue from the marrow cavity of a fossilized leg bone from a Tyrannosaurus rex. The bone had been intentionally, though reluctantly, broken for shipping and then not preserved in the normal manner, specifically because Schweitzer was hoping to test it for soft tissue.[103] Designated as the Museum of the Rockies specimen 1125, or MOR 1125, the dinosaur was previously excavated from the Hell Creek Formation. Flexible, bifurcating blood vessels and fibrous but elastic bone matrix tissue were recognized. In addition, microstructures resembling blood cells were found inside the matrix and vessels. The structures bear resemblance to ostrich blood cells and vessels. Whether an unknown process, distinct from normal fossilization, preserved the material, or the material is original, the researchers do not know, and they are careful not to make any claims about preservation.[104] If it is found to be original material, any surviving proteins may be used as a means of indirectly guessing some of the DNA content of the dinosaurs involved, because each protein is typically created by a specific gene. The absence of previous finds may be the result of people assuming preserved tissue was impossible, therefore not looking. Since the first, two more tyrannosaurs and a hadrosaur have also been found to have such tissue-like structures.[103] Research on some of the tissues involved has suggested that birds are closer relatives to tyrannosaurs than other modern animals.[105]
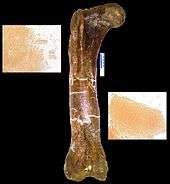
In studies reported in Science in April 2007, Asara and colleagues concluded that seven traces of collagen proteins detected in purified Tyrannosaurus rex bone most closely match those reported in chickens, followed by frogs and newts. The discovery of proteins from a creature tens of millions of years old, along with similar traces the team found in a mastodon bone at least 160,000 years old, upends the conventional view of fossils and may shift paleontologists' focus from bone hunting to biochemistry. Until these finds, most scientists presumed that fossilization replaced all living tissue with inert minerals. Paleontologist Hans Larsson of McGill University in Montreal, who was not part of the studies, called the finds "a milestone", and suggested that dinosaurs could "enter the field of molecular biology and really slingshot paleontology into the modern world".[106]
The presumed soft tissue was called into question by Thomas Kaye of the University of Washington and his co-authors in 2008. They contend that what was really inside the tyrannosaur bone was slimy biofilm created by bacteria that coated the voids once occupied by blood vessels and cells.[107] The researchers found that what previously had been identified as remnants of blood cells, because of the presence of iron, were actually framboids, microscopic mineral spheres bearing iron. They found similar spheres in a variety of other fossils from various periods, including an ammonite. In the ammonite they found the spheres in a place where the iron they contain could not have had any relationship to the presence of blood.[108] Schweitzer has strongly criticized Kaye's claims and argues that there is no reported evidence that biofilms can produce branching, hollow tubes like those noted in her study.[109] San Antonio, Schweitzer and colleagues published an analysis in 2011 of what parts of the collagen had been recovered, finding that it was the inner parts of the collagen coil that had been preserved, as would have been expected from a long period of protein degradation.[110] Other research challenges the identification of soft tissue as biofilm and confirms finding "branching, vessel-like structures" from within fossilized bone.[111]
Thermoregulation

As of 2014, it is not clear if Tyrannosaurus was endothermic (“warm-blooded”). Tyrannosaurus, like most dinosaurs, was long thought to have an ectothermic ("cold-blooded") reptilian metabolism. The idea of dinosaur ectothermy was challenged by scientists like Robert T. Bakker and John Ostrom in the early years of the "Dinosaur Renaissance", beginning in the late 1960s.[112][113] Tyrannosaurus rex itself was claimed to have been endothermic ("warm-blooded"), implying a very active lifestyle.[9] Since then, several paleontologists have sought to determine the ability of Tyrannosaurus to regulate its body temperature. Histological evidence of high growth rates in young Tyrannosaurus rex, comparable to those of mammals and birds, may support the hypothesis of a high metabolism. Growth curves indicate that, as in mammals and birds, Tyrannosaurus rex growth was limited mostly to immature animals, rather than the indeterminate growth seen in most other vertebrates.[79]
Oxygen isotope ratios in fossilized bone are sometimes used to determine the temperature at which the bone was deposited, as the ratio between certain isotopes correlates with temperature. In one specimen, the isotope ratios in bones from different parts of the body indicated a temperature difference of no more than 4 to 5 °C (7 to 9 °F) between the vertebrae of the torso and the tibia of the lower leg. This small temperature range between the body core and the extremities was claimed by paleontologist Reese Barrick and geochemist William Showers to indicate that Tyrannosaurus rex maintained a constant internal body temperature (homeothermy) and that it enjoyed a metabolism somewhere between ectothermic reptiles and endothermic mammals.[114] Other scientists have pointed out that the ratio of oxygen isotopes in the fossils today does not necessarily represent the same ratio in the distant past, and may have been altered during or after fossilization (diagenesis).[115] Barrick and Showers have defended their conclusions in subsequent papers, finding similar results in another theropod dinosaur from a different continent and tens of millions of years earlier in time (Giganotosaurus).[116] Ornithischian dinosaurs also showed evidence of homeothermy, while varanid lizards from the same formation did not.[117] Even if Tyrannosaurus rex does exhibit evidence of homeothermy, it does not necessarily mean that it was endothermic. Such thermoregulation may also be explained by gigantothermy, as in some living sea turtles.[118][119][120] Similar to contemporary alligators, dorsotemporal fenestra in Tyrannosaurus's skull may have aided thermoregulation.[121]
Speed

Scientists have produced a wide range of possible maximum running speeds for Tyrannosaurus, mostly around 11 meters per second (40 km/h; 25 mph), but as low as 5–11 meters per second (18–40 km/h; 11–25 mph) and as high as 20 meters per second (72 km/h; 45 mph). Estimates that Tyrannosaurus had relatively larger leg muscles than any animal alive today indicate that fast running was possible at speeds of 40–70 kilometers per hour (25–43 mph).[122] Researchers have relied on various estimating techniques because, while there are many tracks of large theropods walking, none had the pattern of running.[123]
A 2002 report used a mathematical model (validated by applying it to three living animals: alligators, chickens, and humans; and eight more species, including emus and ostriches[123]) to gauge the leg muscle mass needed for fast running (over 40 km/h or 25 mph).[122] Scientists who think that Tyrannosaurus was able to run point out that hollow bones and other features that would have lightened its body may have kept adult weight to a mere 4.5 metric tons (5.0 short tons) or so, or that other animals like ostriches and horses with long, flexible legs are able to achieve high speeds through slower but longer strides.[123] Proposed top speeds exceeded 40 kilometers per hour (25 mph) for Tyrannosaurus, but were deemed infeasible because they would require exceptional leg muscles of approximately 40–86% of total body mass. Even moderately fast speeds would have required large leg muscles. If the muscle mass was less, only 18 kilometers per hour (11 mph) for walking or jogging would have been possible.[122] Holtz noted that tyrannosaurids and some closely related groups had significantly longer distal hindlimb components (shin plus foot plus toes) relative to the femur length than most other theropods, and that tyrannosaurids and their close relatives had a tightly interlocked metatarsus (foot bones).[124] The third metatarsal was squeezed between the second and fourth metatarsals to form a single unit called an arctometatarsus. This ankle feature may have helped the animal to run more efficiently.[125] Together, these leg features allowed Tyrannosaurus to transmit locomotory forces from the foot to the lower leg more effectively than in earlier theropods.[124]
A 2017 study estimated the top running speed of Tyrannosaurus as 17 mph (27 km/h), speculating that Tyrannosaurus exhausted its energy reserves long before reaching top speed, resulting in a parabola-like relationship between size and speed.[126][127] Another 2017 study hypothesized that an adult Tyrannosaurus was incapable of running due to high skeletal loads. Using a calculated weight estimate of 7 tons, the model showed that speeds above 11 mph (18 km/h) would have probably shattered the leg bones of Tyrannosaurus. The finding may mean that running was also not possible for other giant theropod dinosaurs like Giganotosaurus, Mapusaurus and Acrocanthosaurus.[128] However, studies by Eric Snively et al., published in 2019 indicate that Tyrannosaurus and other tyrannosaurids were more maneuverable than allosauroids and other theropods of comparable size due to low rotational inertia compared to their body mass combined with large leg muscles. As a result, it is hypothesized that Tyrannosaurus was capable of making relatively quick turns and could likely pivot its body more quickly when close to its prey, or that while turning, the theropod could "pirouette" on a single planted foot while the alternating leg was held out in a suspended swing during pursuit. The results of this study potentially could shed light on how agility could have contributed to the success of tyrannosaurid evolution.[129]
Brain and senses
A study conducted by Lawrence Witmer and Ryan Ridgely of Ohio University found that Tyrannosaurus shared the heightened sensory abilities of other coelurosaurs, highlighting relatively rapid and coordinated eye and head movements, as well as an enhanced ability to sense low frequency sounds that would allow tyrannosaurs to track prey movements from long distances and an enhanced sense of smell.[130] A study published by Kent Stevens concluded that Tyrannosaurus had keen vision. By applying modified perimetry to facial reconstructions of several dinosaurs including Tyrannosaurus, the study found that Tyrannosaurus had a binocular range of 55 degrees, surpassing that of modern hawks. Stevens also estimated that Tyrannosaurus had 13 times the visual acuity of a human, thereby surpassing the visual acuity of an eagle which is 3.6 times that of a person, and a limiting far point (that is the distance at which an object can be seen as separate from the horizon) as far as 6 km (3.7 mi) away, which is greater than the 1.6 km (1 mi) that a human can see.[15][16][131]
Thomas Holtz Jr. would note that high depth perception of Tyrannosaurus may have been due to the prey it had to hunt, noting that it had to hunt horned dinosaurs such as Triceratops, armored dinosaurs such as Ankylosaurus, and the duck-billed dinosaurs and their possibly complex social behaviors. He would suggest that this made precision more crucial for Tyrannosaurus enabling it to, "get in, get that blow in and take it down." In contrast, Acrocanthosaurus had limited depth perception because they hunted large sauropods, which were relatively rare during the time of Tyrannosaurus.[86]

Tyrannosaurus had very large olfactory bulbs and olfactory nerves relative to their brain size, the organs responsible for a heightened sense of smell. This suggests that the sense of smell was highly developed, and implies that tyrannosaurs could detect carcasses by scent alone across great distances. The sense of smell in tyrannosaurs may have been comparable to modern vultures, which use scent to track carcasses for scavenging. Research on the olfactory bulbs has shown that Tyrannosaurus rex had the most highly developed sense of smell of 21 sampled non-avian dinosaur species.[132]
Somewhat unusually among theropods, T. rex had a very long cochlea. The length of the cochlea is often related to hearing acuity, or at least the importance of hearing in behavior, implying that hearing was a particularly important sense to tyrannosaurs. Specifically, data suggests that Tyrannosaurus rex heard best in the low-frequency range, and that low-frequency sounds were an important part of tyrannosaur behavior.[130] A 2017 study by Thomas Carr and colleagues found that the snout of tyrannosaurids was highly sensitive, based on a high number of small openings in the facial bones of the related Daspletosaurus that contained sensory neurons. The study speculated that tyrannosaurs might have used their sensitive snouts to measure the temperature of their nests and to gently pick-up eggs and hatchlings, as seen in modern crocodylians.[32]
A study by Grant R. Hurlburt, Ryan C. Ridgely and Lawrence Witmer obtained estimates for Encephalization Quotients (EQs), based on reptiles and birds, as well as estimates for the ratio of cerebrum to brain mass. The study concluded that Tyrannosaurus had the relatively largest brain of all adult non-avian dinosaurs with the exception of certain small maniraptoriforms (Bambiraptor, Troodon and Ornithomimus). The study found that Tyrannosaurus's relative brain size was still within the range of modern reptiles, being at most 2 standard deviations above the mean of non-avian reptile EQs. The estimates for the ratio of cerebrum mass to brain mass would range from 47.5 to 49.53 percent. According to the study, this is more than the lowest estimates for extant birds (44.6 percent), but still close to the typical ratios of the smallest sexually mature alligators which range from 45.9–47.9 percent.[133] Other studies, such as those by Steve Brusatte, indicate the encephalization quotient of Tyrannosaurus was similar in range (2.0-2.4) to a chimpanzee (2.2-2.5), though this may be debatable as reptilian and mammalian encephalization quotients are not equivalent.[134]
Feeding strategies
Most paleontologists accept that Tyrannosaurus was both an active predator and a scavenger like most large carnivores.[135] By far the largest carnivore in its environment, Tyrannosaurus rex was most likely an apex predator, preying upon hadrosaurs, armored herbivores like ceratopsians and ankylosaurs, and possibly sauropods.[136] A study in 2012 by Karl Bates and Peter Falkingham found that Tyrannosaurus had the most powerful bite of any terrestrial animal that has ever lived, finding an adult Tyrannosaurus could have exerted 35,000 to 57,000 N (7,868 to 12,814 lbf) of force in the back teeth.[137][138][139] Even higher estimates were made by Mason B. Meers in 2003.[18] This allowed it to crush bones during repetitive biting and fully consume the carcasses of large dinosaurs.[51] Stephan Lautenschlager and colleagues calculated that Tyrannosaurus was capable of a maximum jaw gape of around 80 degrees, a necessary adaptation for a wide range of jaw angles to power the creature's strong bite.[140][141]
A debate exists, however, about whether Tyrannosaurus was primarily a predator or a pure scavenger; the debate was assessed in a 1917 study by Lambe which argued Tyrannosaurus was a pure scavenger because the Gorgosaurus teeth showed hardly any wear.[142] This argument may not be valid because theropods replaced their teeth quite rapidly. Ever since the first discovery of Tyrannosaurus most scientists have speculated that it was a predator; like modern large predators it would readily scavenge or steal another predator's kill if it had the opportunity.[143]
Paleontologist Jack Horner has been a major proponent of the view that Tyrannosaurus was not a predator at all but instead was exclusively a scavenger.[99][144][145] He has put forward arguments in the popular literature to support the pure scavenger hypothesis:
- Tyrannosaur arms are short when compared to other known predators. Horner argues that the arms were too short to make the necessary gripping force to hold on to prey.[145]
- Tyrannosaurs had large olfactory bulbs and olfactory nerves (relative to their brain size). These suggest a highly developed sense of smell which could sniff out carcasses over great distances, as modern vultures do. Research on the olfactory bulbs of dinosaurs has shown that Tyrannosaurus had the most highly developed sense of smell of 21 sampled dinosaurs.[132]
- Tyrannosaur teeth could crush bone, and therefore could extract as much food (bone marrow) as possible from carcass remnants, usually the least nutritious parts. Karen Chin and colleagues have found bone fragments in coprolites (fossilized feces) that they attribute to tyrannosaurs, but point out that a tyrannosaur's teeth were not well adapted to systematically chewing bone like hyenas do to extract marrow.[146]
- Since at least some of Tyrannosaurus's potential prey could move quickly, evidence that it walked instead of ran could indicate that it was a scavenger.[144] On the other hand, recent analyses suggest that Tyrannosaurus, while slower than large modern terrestrial predators, may well have been fast enough to prey on large hadrosaurs and ceratopsians.[122][54]
Other evidence suggests hunting behavior in Tyrannosaurus. The eye sockets of tyrannosaurs are positioned so that the eyes would point forward, giving them binocular vision slightly better than that of modern hawks. It is not obvious why natural selection would have favored this long-term trend if tyrannosaurs had been pure scavengers, which would not have needed the advanced depth perception that stereoscopic vision provides.[15][16] In modern animals, binocular vision is found mainly in predators.

A skeleton of the hadrosaurid Edmontosaurus annectens has been described from Montana with healed tyrannosaur-inflicted damage on its tail vertebrae. The fact that the damage seems to have healed suggests that the Edmontosaurus survived a tyrannosaur's attack on a living target, i.e. the tyrannosaur had attempted active predation.[147] There is also evidence for an aggressive interaction between a Triceratops and a Tyrannosaurus in the form of partially healed tyrannosaur tooth marks on a Triceratops brow horn and squamosal (a bone of the neck frill); the bitten horn is also broken, with new bone growth after the break. It is not known what the exact nature of the interaction was, though: either animal could have been the aggressor.[148] Since the Triceratops wounds healed, it is most likely that the Triceratops survived the encounter and managed to overcome the Tyrannosaurus. In a battle against a bull Triceratops, the Triceratops would likely defend itself by inflicting fatal wounds to the Tyrannosaurus using its sharp horns.[149] Studies of Sue found a broken and healed fibula and tail vertebrae, scarred facial bones and a tooth from another Tyrannosaurus embedded in a neck vertebra, providing evidence for aggressive behavior.[150] Studies on hadrosaur vertebrae from the Hell Creek Formation that were punctured by the teeth of what appears to be a late-stage juvenile Tyrannosaurus indicate that despite lacking the bone-crushing adaptations of the adults, young individuals were still capable of using the same bone-puncturing feeding technique as their adult counterparts.[151]

Tyrannosaurus may have had infectious saliva used to kill its prey, as proposed by William Abler in 1992. Abler observed that the serrations (tiny protuberances) on the cutting edges of the teeth are closely spaced, enclosing little chambers. These chambers might have trapped pieces of carcass with bacteria, giving Tyrannosaurus a deadly, infectious bite much like the Komodo dragon was thought to have.[152][153] Jack Horner and Don Lessem, in a 1993 popular book, questioned Abler's hypothesis, arguing that Tyrannosaurus's tooth serrations as more like cubes in shape than the serrations on a Komodo monitor's teeth, which are rounded.[99]:214–215
Tyrannosaurus, and most other theropods, probably primarily processed carcasses with lateral shakes of the head, like crocodilians. The head was not as maneuverable as the skulls of allosauroids, due to flat joints of the neck vertebrae.[154]
Social behavior

Suggesting that Tyrannosaurus may have been pack hunters, Philip J. Currie compared T. rex to related species Tarbosaurus bataar and Albertosaurus sarcophagus, citing fossil evidence that may indicate pack behavior.[155] A find in South Dakota where three T. rex skeletons were in close proximity suggested a pack.[156][157] Because available prey such as Triceratops and Ankylosaurus had significant defenses, it may have been effective for T. rex to hunt in groups.[155]
Currie's pack-hunting hypothesis has been criticized for not having been peer-reviewed, but rather was discussed in a television interview and book called Dino Gangs.[158] The Currie theory for pack hunting by T. rex is based mainly by analogy to a different species, Tarbosaurus bataar, and that the supposed evidence for pack hunting in T. bataar itself had not yet been peer-reviewed. According to scientists assessing the Dino Gangs program, the evidence for pack hunting in Tarbosaurus and Albertosaurus is weak and based on skeletal remains for which alternate explanations may apply (such as drought or a flood forcing dinosaurs to die together in one place).[158] Fossilized trackways from the Upper Cretaceous Wapiti Formation of northeastern British Columbia, Canada, left by three tyrannosaurids traveling in the same direction, may also indicate packs.[159][160]
Evidence of intraspecific attack were found by Joseph Peterson and his colleagues in the juvenile Tyrannosaurus nicknamed Jane. Peterson and his team found that Jane's skull showed healed puncture wounds on the upper jaw and snout which they believe came from another juvenile Tyrannosaurus. Subsequent CT scans of Jane's skull would further confirm the team's hypothesis, showing that the puncture wounds came from a traumatic injury and that there was subsequent healing.[161] The team would also state that Jane's injuries were structurally different from the parasite-induced lesions found in Sue and that Jane's injuries were on her face whereas the parasite that infected Sue caused lesions to the lower jaw.[162]
Pathology

In 2001, Bruce Rothschild and others published a study examining evidence for stress fractures and tendon avulsions in theropod dinosaurs and the implications for their behavior. Since stress fractures are caused by repeated trauma rather than singular events they are more likely to be caused by regular behavior than other types of injuries. Of the 81 Tyrannosaurus foot bones examined in the study one was found to have a stress fracture, while none of the 10 hand bones were found to have stress fractures. The researchers found tendon avulsions only among Tyrannosaurus and Allosaurus. An avulsion injury left a divot on the humerus of Sue the T. rex, apparently located at the origin of the deltoid or teres major muscles. The presence of avulsion injuries being limited to the forelimb and shoulder in both Tyrannosaurus and Allosaurus suggests that theropods may have had a musculature more complex than and functionally different from those of birds. The researchers concluded that Sue's tendon avulsion was probably obtained from struggling prey. The presence of stress fractures and tendon avulsions in general provides evidence for a "very active" predation-based diet rather than obligate scavenging.[163]
A 2009 study showed that smooth-edged holes in the skulls of several specimens might have been caused by Trichomonas-like parasites that commonly infect birds. Seriously infected individuals, including "Sue" and MOR 980 ("Peck's Rex"), might therefore have died from starvation after feeding became increasingly difficult. Previously, these holes had been explained by the bacterious bone infection Actinomycosis or by intraspecific attacks.[164]
One study of Tyrannosaurus specimens with tooth marks in the bones attributable to the same genus was presented as evidence of cannibalism.[165] Tooth marks in the humerus, foot bones and metatarsals, may indicate opportunistic scavenging, rather than wounds caused by combat with another T. rex.[165][166] Other tyrannosaurids may also have practiced cannibalism.[165]
Paleoecology
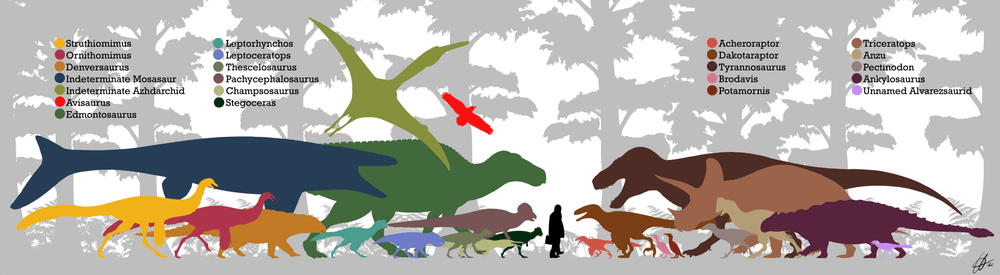
Tyrannosaurus lived during what is referred to as the Lancian faunal stage (Maastrichtian age) at the end of the Late Cretaceous. Tyrannosaurus ranged from Canada in the north to at least New Mexico in the south of Laramidia.[30] During this time Triceratops was the major herbivore in the northern portion of its range, while the titanosaurian sauropod Alamosaurus "dominated" its southern range. Tyrannosaurus remains have been discovered in different ecosystems, including inland and coastal subtropical, and semi-arid plains.
Several notable Tyrannosaurus remains have been found in the Hell Creek Formation. During the Maastrichtian this area was subtropical, with a warm and humid climate. The flora consisted mostly of angiosperms, but also included trees like dawn redwood (Metasequoia) and Araucaria. Tyrannosaurus shared this ecosystem with ceratopsians Leptoceratops, Torosaurus, and Triceratops, the hadrosaurid Edmontosaurus annectens, the parksosaurid Thescelosaurus, the ankylosaurs Ankylosaurus and Denversaurus, the pachycephalosaurs Pachycephalosaurus and Sphaerotholus, and the theropods Ornithomimus, Struthiomimus, Acheroraptor, Dakotaraptor, Pectinodon and Anzu.[167]
Another formation with Tyrannosaurus remains is the Lance Formation of Wyoming. This has been interpreted as a bayou environment similar to today's Gulf Coast. The fauna was very similar to Hell Creek, but with Struthiomimus replacing its relative Ornithomimus. The small ceratopsian Leptoceratops also lived in the area.[168]
In its southern range Tyrannosaurus lived alongside the titanosaur Alamosaurus, the ceratopsians Torosaurus, Bravoceratops and Ojoceratops, hadrosaurs which consisted of a species of Edmontosaurus, Kritosaurus and a possible species of Gryposaurus, the nodosaur Glyptodontopelta, the oviraptorid Ojoraptosaurus, possible species of the theropods Troodon and Richardoestesia, and the pterosaur Quetzalcoatlus.[169] The region is thought to have been dominated by semi-arid inland plains, following the probable retreat of the Western Interior Seaway as global sea levels fell.[170]
Tyrannosaurus may have also inhabited Mexico's Lomas Coloradas formation in Sonora. Though skeletal evidence is lacking, six shed and broken teeth from the fossil bed have been thoroughly compared with other theropod genera and appear to be identical to those of Tyrannosaurus. If true, the evidence indicates the range of Tyrannosaurus was possibly more extensive than previously believed.[171] It is possible that tyrannosaurs were originally Asian species, migrating to North America before the end of the Cretaceous period.[172]
Cultural significance
Since it was first described in 1905, Tyrannosaurus rex has become the most widely recognized dinosaur species in popular culture. It is the only dinosaur that is commonly known to the general public by its full scientific name (binomial name) and the scientific abbreviation T. rex has also come into wide usage.[21] Robert T. Bakker notes this in The Dinosaur Heresies and explains that, "a name like ‘Tyrannosaurus rex’ is just irresistible to the tongue."[9]
References
Notes
- Pronounced /tɪˌrænəˈsɔːrəs, taɪ-/, meaning "tyrant lizard", from the Greek tyrannos (τύραννος), "tyrant", and sauros (σαῦρος), "lizard"[1]
Citations
- "Tyrannosaurus". Online Etymology Dictionary.
- "A T. rex Named Sue" (PDF). The Field Museum. The Field Museum. Archived from the original (PDF) on August 18, 2016. Retrieved January 4, 2019.
- Hutchinson, J. R.; Bates, K. T.; Molnar, J.; Allen, V.; Makovicky, P. J. (2011). "A Computational Analysis of Limb and Body Dimensions in Tyrannosaurus rex with Implications for Locomotion, Ontogeny, and Growth". PLOS One. 6 (10): e26037. Bibcode:2011PLoSO...626037H. doi:10.1371/journal.pone.0026037. PMC 3192160. PMID 22022500.
- "Sue Fact Sheet" (PDF). Sue at the Field Museum. Field Museum of Natural History. Archived from the original (PDF) on August 18, 2016.
- Therrien, F.; Henderson, D. M. (March 12, 2007). "My theropod is bigger than yours … or not: estimating body size from skull length in theropods". Journal of Vertebrate Paleontology. 27 (1): 108–115. doi:10.1671/0272-4634(2007)27[108:MTIBTY]2.0.CO;2. ISSN 0272-4634.
- Persons, S. W.; Currie, P. J.; Erickson, G. M. (2020). "An Older and Exceptionally Large Adult Specimen of Tyrannosaurus rex". The Anatomical Record. 303 (4): 656–672. doi:10.1002/ar.24118. ISSN 1932-8486. PMID 30897281.
- Lyle, A. (March 22, 2019). "Paleontologists identify biggest Tyrannosaurus rex ever discovered". Folio, University of Alberta. Retrieved March 25, 2019.
- Anderson, J. F.; Hall-Martin, A. J.; Russell, D. (1985). "Long bone circumference and weight in mammals, birds and dinosaurs". Journal of Zoology. 207 (1): 53–61. Bibcode:2010JZoo..281..263G. doi:10.1111/j.1469-7998.1985.tb04915.x.
- Bakker, R. T. (1986). The Dinosaur Heresies. New York: Kensington Publishing. p. 241. ISBN 978-0-688-04287-5. OCLC 13699558.
- Henderson, D. M. (January 1, 1999). "Estimating the masses and centers of mass of extinct animals by 3-D mathematical slicing". Paleobiology. 25 (1): 88–106.
- Erickson, G. M.; Makovicky, P. J.; Currie, P. J.; Norell, M. A.; Yerby, S. A.; Brochu, C. A. (2004). "Gigantism and comparative life-history parameters of tyrannosaurid dinosaurs". Nature. 430 (7001): 772–775. Bibcode:2004Natur.430..772E. doi:10.1038/nature02699. PMID 15306807.
- Farlow, J. O.; Smith, M. B.; Robinson, J. M. (1995). "Body mass, bone 'strength indicator', and cursorial potential of Tyrannosaurus rex". Journal of Vertebrate Paleontology. 15 (4): 713–725. doi:10.1080/02724634.1995.10011257. Archived from the original on October 23, 2008.
- Seebacher, F. (2001). "A new method to calculate allometric length–mass relationships of dinosaurs" (PDF). Journal of Vertebrate Paleontology. 21 (1): 51–60. CiteSeerX 10.1.1.462.255. doi:10.1671/0272-4634(2001)021[0051:ANMTCA]2.0.CO;2.
- Christiansen, P.; Fariña, R. A. (2004). "Mass prediction in theropod dinosaurs". Historical Biology. 16 (2–4): 85–92. doi:10.1080/08912960412331284313.
- Stevens, Kent A. (June 2006). "Binocular vision in theropod dinosaurs". Journal of Vertebrate Paleontology. 26 (2): 321–330. doi:10.1671/0272-4634(2006)26[321:BVITD]2.0.CO;2.
- Jaffe, E. (July 1, 2006). "Sight for 'Saur Eyes: T. rex vision was among nature's best". Science News. 170 (1): 3–4. doi:10.2307/4017288. JSTOR 4017288. Retrieved October 6, 2008.
- Snively, E.; Henderson, D. M.; Phillips, D. S. (2006). "Fused and vaulted nasals of tyrannosaurid dinosaurs: Implications for cranial strength and feeding mechanics" (PDF). Acta Palaeontologica Polonica. 51 (3): 435–454. Retrieved October 8, 2008.
- Meers, M. B. (August 2003). "Maximum bite force and prey size of Tyrannosaurus rex and their relationships to the inference of feeding behavior". Historical Biology. 16 (1): 1–12. doi:10.1080/0891296021000050755.
- Erickson, G. M.; Van Kirk, S. D.; Su, J.; Levenston, M. E.; Caler, W. E.; Carter, D. R. (1996). "Bite-force estimation for Tyrannosaurus rex from tooth-marked bones". Nature. 382 (6593): 706–708. Bibcode:1996Natur.382..706E. doi:10.1038/382706a0.
- Holtz, T. R. (1994). "The Phylogenetic Position of the Tyrannosauridae: Implications for Theropod Systematics". Journal of Paleontology. 68 (5): 1100–1117. doi:10.1017/S0022336000026706. JSTOR 1306180.
- Brochu, C. R. (2003). "Osteology of Tyrannosaurus rex: insights from a nearly complete skeleton and high-resolution computed tomographic analysis of the skull". Society of Vertebrate Paleontology Memoirs. 7: 1–138. doi:10.2307/3889334. JSTOR 3889334.
- Smith, J. B. (December 1, 2005). "Heterodonty in Tyrannosaurus rex: implications for the taxonomic and systematic utility of theropod dentitions". Journal of Vertebrate Paleontology. 25 (4): 865–887. doi:10.1671/0272-4634(2005)025[0865:HITRIF]2.0.CO;2.
- Douglas, K.; Young, S. (1998). "The dinosaur detectives". New Scientist. Retrieved October 16, 2008.
One palaeontologist memorably described the huge, curved teeth of T. rex as 'lethal bananas'
- "Sue's vital statistics". Sue at the Field Museum. Field Museum of Natural History. Archived from the original on September 29, 2007. Retrieved September 15, 2007.
- Lipkin, C.; Carpenter, K. (2008). "Looking again at the forelimb of Tyrannosaurus rex". In Carpenter, K.; Larson, P. E. (eds.). Tyrannosaurus rex, the Tyrant King. Bloomington: Indiana University Press. pp. 167–190. ISBN 978-0-253-35087-9.
- Bell, P. R.; Campione, N. E.; Persons IV, W. S.; Currie, P. J.; Larson, P. L.; Tanke, D. H.; Bakker, R. T. (2017). "Tyrannosauroid integument reveals conflicting patterns of gigantism and feather evolution". Biology Letters. 13 (6): 20170092. doi:10.1098/rsbl.2017.0092. PMC 5493735. PMID 28592520.
- Farago, J. (March 7, 2019). "T. Rex Like You Haven't Seen Him: With Feathers". The New York Times. Retrieved March 7, 2019.
- Xing, X.; Norell, M. A.; Kuang, X.; Wang, X.; Zhao, Q.; Jia, C. (October 7, 2004). "Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids". Nature. 431 (7009): 680–684. Bibcode:2004Natur.431..680X. doi:10.1038/nature02855. PMID 15470426.
- Xing, X.; Wang, K.; Zhang; Ma, Q.; Xing, L.; Sullivan, C.; Hu, D.; Cheng, S.; Wang, S. (April 5, 2012). "A gigantic feathered dinosaur from the Lower Cretaceous of China" (PDF). Nature. 484 (7392): 92–95. Bibcode:2012Natur.484...92X. doi:10.1038/nature10906. PMID 22481363. Archived from the original (PDF) on April 17, 2012.
- Larson, N. L. (2008). "One hundred years of Tyrannosaurus rex: the skeletons". In Larson, P.; Carpenter, K. (eds.). Tyrannosaurus rex, The Tyrant King. Bloomington, IN: Indiana University Press. pp. 1–55. ISBN 978-0-253-35087-9.
- Reisz, R. R.; Larson, D. (2016). "Dental anatomy and skull length to tooth size ratios support the hypothesis that theropod dinosaurs had lips". 4th Annual Meeting, 2016, Canadian Society of Vertebrate Palaeontology. ISSN 2292-1389.
- Carr, T. D.; Varricchio, D. J.; Sedlmayr, J. C.; Roberts, E. M.; Moore, J. R. (March 30, 2017). "A new tyrannosaur with evidence for anagenesis and crocodile-like facial sensory system". Scientific Reports. 7: 44942. Bibcode:2017NatSR...744942C. doi:10.1038/srep44942. ISSN 2045-2322. PMC 5372470. PMID 28358353.
- Naish, D. "The Sensitive Face of a Big Predatory Dinosaur". Tetrapod Zoology. Scientific American Blog Network. Retrieved December 5, 2018.
- Breithaupt, B. H.; Southwell, E. H.; Matthews, N. A. (October 15, 2005). "In Celebration of 100 years of Tyrannosaurus rex: Manospondylus gigas, Ornithomimus grandis, and Dynamosaurus imperiosus, the Earliest Discoveries of Tyrannosaurus rex in the West". Abstracts with Programs; 2005 Salt Lake City Annual Meeting. Geological Society of America. 37 (7): 406. Retrieved October 8, 2008.
- Hatcher, J. B. (1907). "The Ceratopsia". Monographs of the United States Geological Survey. 49: 113–114.
- Osborn, H. F. (1917). "Skeletal adaptations of Ornitholestes, Struthiomimus, Tyrannosaurus". Bulletin of the American Museum of Natural History. 35 (43): 733–771. hdl:2246/1334.
- Osborn, H. F. (1905). "Tyrannosaurus and other Cretaceous carnivorous dinosaurs". Bulletin of the AMNH. 21 (14): 259–265. hdl:2246/1464. Retrieved October 6, 2008.
- Dingus, L.; Norell, M. (May 3, 2010). Barnum Brown: The Man Who Discovered Tyrannosaurus rex. University of California Press. pp. 90, 124. ISBN 978-0-520-94552-4.
- Osborn, H. F.; Brown, B. (1906). "Tyrannosaurus, Upper Cretaceous carnivorous dinosaur". Bulletin of the AMNH. 22 (16): 281–296. hdl:2246/1473.
- Breithaupt, B. H.; Southwell, E. H.; Matthews, N. A. (2006). Lucas, S. G.; Sullivan, R. M. (eds.). "Dynamosaurus imperiosus and the earliest discoveries of Tyrannosaurus rex in Wyoming and the West" (PDF). New Mexico Museum of Natural History and Science Bulletin. 35: 258.
The original skeleton of Dynamosaurus imperiosus (AMNH 5866/BM R7995), together with other T. rex material (including parts of AMNH 973, 5027, and 5881), were sold to the British Museum of Natural History (now The Natural History Museum) in 1960. This material was used in an interesting 'half-mount' display of this dinosaur in London. Currently the material resides in the research collections.
- McDonald, A. T.; Wolfe, D. G.; Dooley Jr, A. C. (2018). "A new tyrannosaurid (Dinosauria: Theropoda) from the Upper Cretaceous Menefee Formation of New Mexico". PeerJ. 6: 6:e5749. doi:10.7717/peerj.5749. PMC 6183510. PMID 30324024.
- "Preparing Sue's bones". Sue at the Field Museum. The Field Museum. 2007. Retrieved October 24, 2014.
- Erickson, G.; Makovicky, P. J.; Currie, P. J.; Norell, M.; Yerby, S.; Brochu, C. A. (May 26, 2004). "Gigantism and life history parameters of tyrannosaurid dinosaurs". Nature. 430 (7001): 772–775. Bibcode:2004Natur.430..772E. doi:10.1038/nature02699. PMID 15306807.
- "Stan". The University of Manchester. September 18, 2010. Archived from the original on September 18, 2010.
- Fiffer, S. (2000). "Jurassic Farce". Tyrannosaurus Sue. W. H. Freeman and Company, New York. pp. 121–122. ISBN 978-0-7167-4017-9.
- "Meet Bucky The Teenage T. Rex". Children's Museum of Indianapolis. July 7, 2014. Archived from the original on December 27, 2014. Retrieved December 2, 2019.
- "Dig pulls up five T. rex specimens". BBC News. October 10, 2000. Retrieved December 13, 2008.
- Currie, P. J.; Hurum, J. H.; Sabath, K. (2003). "Skull structure and evolution in tyrannosaurid dinosaurs" (PDF). Acta Palaeontologica Polonica. 48 (2): 227–234. Retrieved October 8, 2008.
- Black, Riley (October 28, 2015). "Tiny terror: Controversial dinosaur species is just an awkward tween Tyrannosaurus". Smithsonian Magazine. Retrieved December 10, 2018.
- "Museum unveils world's largest T-rex skull". 2006. Archived from the original on April 14, 2006. Retrieved April 7, 2006.
- Gignac, P. M.; Erickson, G. M. (2017). "The biomechanics behind extreme osteophagy in Tyrannosaurus rex". Scientific Reports. 7 (1): 2012. Bibcode:2017NatSR...7.2012G. doi:10.1038/s41598-017-02161-w. PMC 5435714. PMID 28515439.
- Lockley, M. G.; Hunt, A. P. (1994). "A track of the giant theropod dinosaur Tyrannosaurus from close to the Cretaceous/Tertiary boundary, northern New Mexico". Ichnos. 3 (3): 213–218. Bibcode:1998Ichno...6..141C. doi:10.1080/10420949409386390.
- "A Probable Tyrannosaurid Track From the Hell Creek Formation (Upper Cretaceous), Montana, United States". National Museum of History News. 2007. Archived from the original on December 14, 2007. Retrieved December 18, 2007.
- Manning, P. L.; Ott, C.; Falkingham, P. L. (2009). "The first tyrannosaurid track from the Hell Creek Formation (Late Cretaceous), Montana, U.S.A". PALAIOS. 23 (10): 645–647. Bibcode:2008Palai..23..645M. doi:10.2110/palo.2008.p08-030r.
- Smith, S. D.; Persons, W. S.; Xing, L. (2016). "A "Tyrannosaur" trackway at Glenrock, Lance Formation (Maastrichtian), Wyoming". Cretaceous Research. 61 (1): 1–4. doi:10.1016/j.cretres.2015.12.020.
- Perkins, S. (2016). "You could probably have outrun a T. rex". Palaeontology. doi:10.1126/science.aae0270.
- Walton, T. (2016). "Forget all you know from Jurassic Park: For speed, T. rex beats velociraptors". USA Today. Retrieved March 13, 2016.
- Ruiz, J. (2017). "Comments on "A tyrannosaur trackway at Glenrock, Lance Formation (Maastrichtian), Wyoming" (Smith et al., Cretaceous Research, v. 61, pp. 1–4, 2016)". Cretaceous Research. 82: 81–82. doi:10.1016/j.cretres.2017.05.033.
- Holtz, T. R., Jr. (2004). "Tyrannosauroidea". In Weishampel, D. B.; Dodson, P.; Osmólska, H. (eds.). The dinosauria. Berkeley: University of California Press. pp. 111–136. ISBN 978-0-520-24209-8.
- Paul, Gregory S. (1988). Predatory dinosaurs of the world: a complete illustrated guide. New York: Simon and Schuster. p. 228. ISBN 978-0-671-61946-6. OCLC 18350868.
- Maleev, E. A. (1955). translated by F. J. Alcock. "(title in Russian)" [Gigantic carnivorous dinosaurs of Mongolia] (PDF). Doklady Akademii Nauk SSSR (in Russian). 104 (4): 634–637.
- Rozhdestvensky, A. K. (1965). "Growth changes in Asian dinosaurs and some problems of their taxonomy". Paleontological Journal. 3: 95–109.
- Carpenter, K. (1992). "Tyrannosaurids (Dinosauria) of Asia and North America". In Mateer, N. J.; Chen, P.-j. (eds.). Aspects of nonmarine Cretaceous geology. Beijing: China Ocean Press. pp. 250–268. ISBN 978-7-5027-1463-5. OCLC 28260578.
- Carr, T. D.; Williamson, T. E.; Schwimmer, D. R. (2005). "A New Genus and Species of Tyrannosauroid from the Late Cretaceous (Middle Campanian) Demopolis Formation of Alabama". Journal of Vertebrate Paleontology. 25 (1): 119–143. doi:10.1671/0272-4634(2005)025[0119:ANGASO]2.0.CO;2.
- Hurum, J. H.; Sabath, K. (2003). "Giant theropod dinosaurs from Asia and North America: Skulls of Tarbosaurus bataar and Tyrannosaurus rex compared" (PDF). Acta Palaeontologica Polonica. 48 (2): 161–190. Retrieved October 8, 2008.
- Lü, J.; Yi, L.; Brusatte, S. L.; Yang, L.; Li, H.; Chen, L. (May 7, 2014). "A new clade of Asian late Cretaceous long-snouted tyrannosaurids". Nature Communications. 5: 3788. Bibcode:2014NatCo...5.3788L. doi:10.1038/ncomms4788. PMID 24807588.

- "Pinocchio rex dinosaur found in China adds to tyrannosaur family". CBC News. May 7, 2014. Retrieved November 10, 2017.
- Loewen, M. A.; Irmis, R. B.; Sertich, J. J. W.; Currie, P. J.; Sampson, S. D. (2013). Evans, D. C (ed.). "Tyrant Dinosaur Evolution Tracks the Rise and Fall of Late Cretaceous Oceans". PLoS ONE. 8 (11): e79420. Bibcode:2013PLoSO...879420L. doi:10.1371/journal.pone.0079420. PMC 3819173. PMID 24223179.
- Vergano, D. (November 7, 2013). "Newfound "King of Gore" Dinosaur Ruled Before T. Rex". National Geographic. Retrieved November 10, 2017.
- Geggel, L. (February 29, 2016). "T. Rex Was Likely an Invasive Species". Live Science. Retrieved November 10, 2017.
- Urban, M. A.; Lamanna, M. C. (2006). "Evidence of a giant Tyrannosaurid (Dinosauria: Theropoda) from the upper Cretaceous (?Campannian) of Montana" (PDF). Annals of Carnegie Museum. 75 (4): 231–235. doi:10.2992/0097-4463(2006)75[231:EOAGTD]2.0.CO;2. Archived from the original (PDF) on October 27, 2016. Retrieved November 10, 2017.
- Olshevsky, G. (1995). "The origin and evolution of the tyrannosaurids". Kyoryugaku Saizensen [Dino Frontline]. 9–10: 92–119.
- Carr, T. D.; Williamson, T. E. (2004). "Diversity of late Maastrichtian Tyrannosauridae (Dinosauria: Theropoda) from western North America". Zoological Journal of the Linnean Society. 142 (4): 479–523. doi:10.1111/j.1096-3642.2004.00130.x.
- Gilmore, C. W. (1946). "A new carnivorous dinosaur from the Lance Formation of Montana". Smithsonian Miscellaneous Collections. 106: 1–19.
- Bakker, R. T.; Williams, M.; Currie, P. J. (1988). "Nanotyrannus, a new genus of pygmy tyrannosaur, from the latest Cretaceous of Montana". Hunteria. 1 (5): 1–30.
- Carr, T. D. (1999). "Craniofacial ontogeny in Tyrannosauridae (Dinosauria, Theropoda)". Journal of Vertebrate Paleontology. 19 (3): 497–520. doi:10.1080/02724634.1999.10011161.
- Currie, P. J. (2003). "Cranial anatomy of tyrannosaurid dinosaurs from the Late Cretaceous of Alberta, Canada" (PDF). Acta Palaeontologica Polonica. 42 (2): 191–226. Retrieved October 9, 2008.
- Hone, D. W. E.; Wang, K.; Sullivan, C.; Zhao, X.; Chen, S.; Li, D.; Ji, S.; Ji, Q.; Xu, X. (2011). "A new, large tyrannosaurine theropod from the Upper Cretaceous of China". Cretaceous Research. 32 (4): 495–503. doi:10.1016/j.cretres.2011.03.005.
- Horner, J. R.; Padian, K. (2004). "Age and growth dynamics of Tyrannosaurus rex". Proceedings: Biological Sciences. 271 (1551): 1875–80. doi:10.1098/rspb.2004.2829. PMC 1691809. PMID 15347508. Retrieved October 5, 2008.
- Schweitzer, M. H.; Wittmeyer, J. L.; Horner, J. R. (2005). "Gender-specific reproductive tissue in ratites and Tyrannosaurus rex". Science. 308 (5727): 1456–60. Bibcode:2005Sci...308.1456S. doi:10.1126/science.1112158. PMID 15933198.
- Lee, A. H.; Werning, S. (2008). "Sexual maturity in growing dinosaurs does not fit reptilian growth models". Proceedings of the National Academy of Sciences. 105 (2): 582–587. Bibcode:2008PNAS..105..582L. doi:10.1073/pnas.0708903105. PMC 2206579. PMID 18195356.
- Schweitzer, M. H.; Zheng, W.; Zanno, L.; Werning, S.; Sugiyama, T. (2016). "Chemistry supports the identification of gender-specific reproductive tissue in Tyrannosaurus rex". Scientific Reports. 6 (23099): 23099. Bibcode:2016NatSR...623099S. doi:10.1038/srep23099. PMC 4791554. PMID 26975806.
- Erickson, G. M.; Currie, P. J.; Inouye, B. D.; Winn, A. A. (2006). "Tyrannosaur life tables: an example of nonavian dinosaur population biology". Science. 313 (5784): 213–7. Bibcode:2006Sci...313..213E. doi:10.1126/science.1125721. PMID 16840697.
- Woodward, Holly N; Tremaine, Katie; Williams, Scott A; Zanno, Lindsay E; Horner, John R; Myhrvold, Nathan (January 1, 2020). "Growing up Tyrannosaurus rex: Osteohistology refutes the pygmy "Nanotyrannus" and supports ontogenetic niche partitioning in juvenile Tyrannosaurus". Science Advances. 6 (1): eaax6250. Bibcode:2020SciA....6.6250W. doi:10.1126/sciadv.aax6250. PMC 6938697. PMID 31911944.
- Greshko, Michael (January 1, 2020). "These sleek predatory dinosaurs really are teenage T. rex". National Geographic. Retrieved January 2, 2020.
- Holtz, T. R., Jr (March 19, 2013) [Lecture was held March 8, 2013]. The Life and Times of Tyrannosaurus rex, with Dr. Thomas Holtz (Lecture). Kane Hall Room 130 University of Washington Seattle, WA 98195: Burke Museum of Natural History and Culture. Retrieved October 12, 2013.CS1 maint: location (link)
- Paul, G. S. (2008). "Chapter 18: The Extreme Life Style and Habits of the Gigantic Tyrannosaurid Superpredators of the Cretaceous North America and Asia". In Larson, P. L.; Carpenter, K. (eds.). Tyrannosaurus, The Tyrant King. Indiana University Press. pp. 307–345. ISBN 978-0-253-35087-9. Retrieved September 14, 2013.
- Carpenter, K. (1992). "Variation in Tyrannosaurus rex". In Carpenter, K.; Currie, P. J. (eds.). Dinosaur Systematics: Approaches and Perspectives. Cambridge: Cambridge University Press. pp. 141–145. ISBN 978-0-521-43810-0.
- Larson, P. L. (1994). "Tyrannosaurus sex". In Rosenberg, G. D.; Wolberg, D. L. (eds.). Dino Fest. 7. The Paleontological Society Special Publications. pp. 139–155.
- Erickson, G. M.; Kristopher, L. A.; Larson, P. (2005). "Androgynous rex – the utility of chevrons for determining the sex of crocodilians and non-avian dinosaurs". Zoology (Jena, Germany). 108 (4): 277–86. doi:10.1016/j.zool.2005.08.001. PMID 16351976.
- Schweitzer, M. H.; Elsey, R. M.; Dacke, C. G.; Horner, J. R.; Lamm, E. T. (2007). "Do egg-laying crocodilian (Alligator mississippiensis) archosaurs form medullary bone?". Bone. 40 (4): 1152–8. doi:10.1016/j.bone.2006.10.029. PMID 17223615.
- Leidy, J. (1865). "Memoir on the extinct reptiles of the Cretaceous formations of the United States". Smithsonian Contributions to Knowledge. 14: 1–135.
- "Tyrannosaurus". American Museum of Natural History. Archived from the original on December 8, 2008. Retrieved October 16, 2008.
- Newman, B. H. (1970). "Stance and gait in the flesh-eating Tyrannosaurus". Biological Journal of the Linnean Society. 2 (2): 119–123. doi:10.1111/j.1095-8312.1970.tb01707.x.
- "The Age of Reptiles Mural". Yale University. 2008. Retrieved October 16, 2008.
- Ross, R. M.; Duggan-Haas, D.; Allmon, W. D. (2013). "The Posture of Tyrannosaurus rex: Why Do Student Views Lag Behind the Science?". Journal of Geoscience Education. 61 (1): 145–160. Bibcode:2013JGeEd..61..145R. doi:10.5408/11-259.1.
- "Tyrannosaurus Rex: Not a tripod anymore". American Association for the Advancement of Science.
- Lambe, L. M. (1914). "On a new genus and species of carnivorous dinosaur from the Belly River Formation of Alberta, with a description of the skull of Stephanosaurus marginatus from the same horizon". Ottawa Naturalist. 27: 129–135.
- Horner, J. R.; Lessem, D. (1993). The complete T. rex. New York City: Simon & Schuster. ISBN 978-0-671-74185-3.
- "A New View of T. Rex | Smithsonian National Museum of Natural History". web.archive.org. April 13, 2020. Retrieved April 13, 2020.
- Carpenter, K.; Smith, M. (2001). "Forelimb Osteology and Biomechanics of Tyrannosaurus rex". In Tanke, D. H.; Carpenter, K. (eds.). Mesozoic vertebrate life. Bloomington: Indiana University Press. pp. 90–116. ISBN 978-0-253-33907-2.
- Pickrell, J. (November 2, 2017). "T. Rex's Tiny Arms May Have Been Vicious Weapons". National Geographic. Retrieved December 10, 2018.
- Fields, H. (2006). "Dinosaur Shocker". Smithsonian Magazine. Retrieved October 2, 2008.
- Schweitzer, M. H.; Wittmeyer, J. L.; Horner, J. R.; Toporski, J. K. (2005). "Soft-tissue vessels and cellular preservation in Tyrannosaurus rex". Science. 307 (5717): 1952–5. Bibcode:2005Sci...307.1952S. doi:10.1126/science.1108397. PMID 15790853.
- Rincon, P. (April 12, 2007). "Protein links T. rex to chickens". BBC News. Retrieved October 2, 2008.
- Vergano, D. (April 13, 2007). "Yesterday's T. Rex is today's chicken". USA Today. Retrieved October 8, 2008.
- Kaye, T. G.; Gaugler, G.; Sawlowicz, Z. (2008). Stepanova, A. (ed.). "Dinosaurian Soft Tissues Interpreted as Bacterial Biofilms". PLOS One. 3 (7): e2808. Bibcode:2008PLoSO...3.2808K. doi:10.1371/journal.pone.0002808. PMC 2483347. PMID 18665236.
- "New Research Challenges Notion That Dinosaur Soft Tissues Still Survive" (Press release). Newswise. July 24, 2008. Retrieved October 8, 2008.
- "Researchers Debate: Is It Preserved Dinosaur Tissue, or Bacterial Slime?" (Press release). Discover. July 30, 2008. Retrieved September 4, 2008.
- San Antonio, J. D.; Schweitzer, M. H.; Jensen, S. T.; Kalluri, R.; Buckley, M.; Orgel, J. P. R. O. (2011). Van Veen, H. W. (ed.). "Dinosaur Peptides Suggest Mechanisms of Protein Survival". PLOS One. 6 (6): e20381. Bibcode:2011PLoSO...620381S. doi:10.1371/journal.pone.0020381. PMC 3110760. PMID 21687667.
- Peterson, J. E.; Lenczewski, M. E.; Scherer, R. P. (October 12, 2010). "Influence of Microbial Biofilms on the Preservation of Primary Soft Tissue in Fossil and Extant Archosaurs". PLOS One. 5 (10): e13334. Bibcode:2010PLoSO...513334P. doi:10.1371/journal.pone.0013334. PMC 2953520. PMID 20967227.
[T]he interpretation of preserved organic remains as microbial biofilm [is] highly unlikely
- Bakker, R. T. (1968). "The superiority of dinosaurs" (PDF). Discovery. 3 (2): 11–12. Archived from the original (PDF) on September 9, 2006. Retrieved October 7, 2008.
- Bakker, R. T. (1972). "Anatomical and ecological evidence of endothermy in dinosaurs" (PDF). Nature. 238 (5359): 81–85. Bibcode:1972Natur.238...81B. doi:10.1038/238081a0. Archived from the original (PDF) on September 9, 2006. Retrieved October 7, 2008.
- Barrick, R. E.; Showers, W. J. (1994). "Thermophysiology of Tyrannosaurus rex: Evidence from Oxygen Isotopes". Science. 265 (5169): 222–224. Bibcode:1994Sci...265..222B. doi:10.1126/science.265.5169.222. PMID 17750663.
- Trueman, C.; Chenery, C.; Eberth, D. A.; Spiro, B. (2003). "Diagenetic effects on the oxygen isotope composition of bones of dinosaurs and other vertebrates recovered from terrestrial and marine sediments". Journal of the Geological Society. 160 (6): 895–901. Bibcode:2003JGSoc.160..895T. doi:10.1144/0016-764903-019.
- Barrick, R. E.; Showers, W. J. (1999). "Thermophysiology and biology of Giganotosaurus: comparison with Tyrannosaurus". Palaeontologia Electronica. 2 (2). Retrieved October 7, 2008.
- Barrick, R. E.; Stoskopf, M. K.; Showers, W. J. (1999). "Oxygen isotopes in dinosaur bones". In Farlow, J. O.; Brett-Surman, M. K. (eds.). The Complete Dinosaur. Bloomington: Indiana University Press. pp. 474–490. ISBN 978-0-253-21313-6.
- Paladino, F. V.; Spotila, J. R.; Dodson, P. (1999). "A blueprint for giants: modeling the physiology of large dinosaurs". In Farlow, J. O.; Brett-Surman, M. K. (eds.). The Complete Dinosaur. Bloomington: Indiana University Press. pp. 491–504. ISBN 978-0-253-21313-6.
- Chinsamy, A.; Hillenius, W. J. (2004). "Physiology of nonavian dinosaurs". In Weishampel, D. B.; Dodson, P.; Osmólska, H. (eds.). The dinosauria. Berkeley: University of California Press. pp. 643–659. ISBN 978-0-520-24209-8.
- Seymour, R. S. (July 5, 2013). "Maximal Aerobic and Anaerobic Power Generation in Large Crocodiles versus Mammals: Implications for Dinosaur Gigantothermy". PLOS One. 8 (7): e69361. Bibcode:2013PLoSO...869361S. doi:10.1371/journal.pone.0069361. ISSN 1932-6203. PMC 3702618. PMID 23861968.
- "T. Rex had an air conditioner in its head, study suggests". ScienceDaily. Retrieved September 6, 2019.
- Hutchinson, J. R.; Garcia, M. (2002). "Tyrannosaurus was not a fast runner". Nature. 415 (6875): 1018–21. Bibcode:2002Natur.415.1018H. doi:10.1038/4151018a. PMID 11875567.
- Hutchinson, J. R. (2004). "Biomechanical Modeling and Sensitivity Analysis of Bipedal Running Ability. II. Extinct Taxa" (PDF). Journal of Morphology. 262 (1): 441–461. doi:10.1002/jmor.10240. PMID 15352202. Archived from the original (PDF) on October 31, 2008.
- Holtz, T. R. (May 1, 1996). "Phylogenetic taxonomy of the Coelurosauria (Dinosauria; Theropoda)". Journal of Paleontology. 70 (3): 536–538. doi:10.1017/S0022336000038506. Retrieved October 3, 2008.
- Benton, M. (2014). Vertebrate Palaeontology (4th ed.). p. 193. ISBN 978-1118407554.
- "Why Tyrannosaurus was a slow runner and why the largest are not always the fastest". ScienceDaily. July 17, 2017. Retrieved November 10, 2017.
- Hirt, M. R.; Jetz, W.; Rall, B. C.; Brose, U. (2017). "A general scaling law reveals why the largest animals are not the fastest". Nature Ecology & Evolution. 1 (8): 1116–1122. doi:10.1038/s41559-017-0241-4. PMID 29046579.
- Sellers, W. I.; Pond, S. B.; Brassey, C. A.; Manning, P. L.; Bates, K. T. (July 18, 2017). "Investigating the running abilities of Tyrannosaurus rex using stress-constrained multibody dynamic analysis". PeerJ. 5: e3420. doi:10.7717/peerj.3420. ISSN 2167-8359. PMC 5518979. PMID 28740745.
- Cotton, J. R.; Hartman, S. A.; Currie, P. J.; Witmer, L. M.; Russell, A. P.; Holtz, T. R. Jr; Burns, M. E.; Surring, L. A.; Mallison, H.; Henderson, D. M.; O’Brien, H.; Snively, E. (February 21, 2019). "Lower rotational inertia and larger leg muscles indicate more rapid turns in tyrannosaurids than in other large theropods". PeerJ. 7: e6432. doi:10.7717/peerj.6432. PMC 6387760. PMID 30809441.
- Witmer, L. M.; Ridgely, R. C. (2009). "New Insights into the Brain, Braincase, and Ear Region of Tyrannosaurs (Dinosauria, Theropoda), with Implications for Sensory Organization and Behavior". The Anatomical Record. 292 (9): 1266–1296. doi:10.1002/ar.20983. PMID 19711459.
- Stevens, K. A. (April 1, 2011). "The Binocular Vision of Theropod Dinosaurs". Retrieved July 19, 2013.
- "T. Rex brain study reveals a refined 'nose'". Calgary Herald. October 28, 2008. Retrieved October 29, 2008.
- Hurlburt, G. S.; Ridgely, R. C.; Witmer, L. M. (July 5, 2013). "Relative size of brain and cerebrum in Tyrannosaurid dinosaurs: an analysis using brain-endocast quantitative relationships in extant alligators". In Parrish, M. J.; Molnar, R. E.; Currie, P. J.; Koppelhus, E. B. (eds.). Tyrannosaurid Paleobiology. Indiana University Press. pp. 134–154. ISBN 978-0-253-00947-0. Retrieved October 20, 2013.
- Brusatten, Steve (2018). The Rise and Fall of the Dinosaurs. New York, New York: HarperCollins Publishers. p. 219. ISBN 9780062490438.
- "Time to Slay the T. rex Scavenger "Debate"". National Geographic. July 16, 2013. Archived from the original on July 12, 2018.
- Black, Riley (April 13, 2012). "When Tyrannosaurus Chomped Sauropods". Smithsonian Magazine. Smithsonian Media. Retrieved August 24, 2013.
- Black, Riley (2012). "The Tyrannosaurus rexs Dangerous and Deadly Bite". Smithsonian Magazine. Smithsonian Institution.
- Bates, K. T.; Falkingham, P. L. (February 29, 2012). "Estimating maximum bite performance in Tyrannosaurus rex using multi-body dynamics". Biological Letters. 8 (4): 660–664. doi:10.1098/rsbl.2012.0056. PMC 3391458. PMID 22378742.
- Scully, C. (2002). Oxford Handbook of Applied Dental Sciences. Oxford University Press. p. 156. ISBN 978-0-19-851096-3.
- Lautenschlager, Stephan (November 4, 2015). "Estimating cranial musculoskeletal constraints in theropod dinosaurs". Royal Society Open Science. 2 (11): 150495. Bibcode:2015RSOS....250495L. doi:10.1098/rsos.150495. PMC 4680622. PMID 26716007.
- "The better to eat you with? How dinosaurs' jaws influenced diet". Science Daily. November 3, 2015. Archived from the original on November 4, 2015. Retrieved September 14, 2018.
- Lambe, L. B. (1917). "The Cretaceous theropodous dinosaur Gorgosaurus". Memoirs of the Geological Survey of Canada. 100: 1–84. doi:10.4095/101672.
- Farlow, J. O.; Holtz (2002). "The fossil record of predation in dinosaurs" (PDF). In Kowalewski, M.; Kelley, P. H. (eds.). The Fossil Record of Predation. The Paleontological Society Papers. 8. T. R. Jr. pp. 251–266. Archived from the original (PDF) on October 31, 2008.
- Horner, J. R. (1994). "Steak knives, beady eyes, and tiny little arms (a portrait of Tyrannosaurus as a scavenger)". The Paleontological Society Special Publication. 7: 157–164. doi:10.1017/S2475262200009497.
- Amos, J. (July 31, 2003). "Science/Nature: T. rex goes on trial". BBC News. Retrieved December 23, 2015.
- Chin, K.; Tokaryk, T. T.; Erickson, G. M.; Calk, L. C. (June 18, 1998). "A king-sized theropod coprolite". Nature. 393 (6686): 680–682. Bibcode:1998Natur.393..680C. doi:10.1038/31461. Summary at Monastersky, R. (June 20, 1998). "Getting the scoop from the poop of T. rex". Science News. 153 (25): 391. doi:10.2307/4010364. JSTOR 4010364. Archived from the original on May 11, 2013.
- Carpenter, K. (1998). "Evidence of predatory behavior by theropod dinosaurs". Gaia. 15: 135–144. Archived from the original on November 17, 2007. Retrieved December 5, 2007.
- Happ, J.; Carpenter, K. (2008). "An analysis of predator–prey behavior in a head-to-head encounter between Tyrannosaurus rex and Triceratops". In Carpenter, K.; Larson, P. E. (eds.). Tyrannosaurus rex, the Tyrant King (Life of the Past). Bloomington: Indiana University Press. pp. 355–368. ISBN 978-0-253-35087-9.
- Dodson, P. The Horned Dinosaurs. Princeton University Press. p. 19.
- Tanke, D. H.; Currie, P. J. (1998). "Head-biting behavior in theropod dinosaurs: paleopathological evidence" (PDF). Gaia (15): 167–184. ISSN 0871-5424. Archived from the original (PDF) on February 27, 2008.
- Peterson, J. E.; Daus, K. N. (March 4, 2019). "Feeding traces attributable to juvenile Tyrannosaurus rex offer insight into ontogenetic dietary trends". PeerJ. 7: e6573. doi:10.7717/peerj.6573. ISSN 2167-8359. PMC 6404657. PMID 30863686.
- Abler, W. L. (1992). "The serrated teeth of tyrannosaurid dinosaurs, and biting structures in other animals". Paleobiology. 18 (2): 161–183. doi:10.1017/S0094837300013956.
- Goldstein, E. J. C.; Tyrrell, K. L.; Citron, D. M.; Cox, C. R.; Recchio, I. M.; Okimoto, B.; Bryja, J.; Fry, B. G. (June 1, 2013). "Anaerobic and aerobic bacteriology of the saliva and gingiva from 16 captive Komodo dragons (Varanus komodoensis): new implications for the "bacteria as venom" model". Journal of Zoo and Wildlife Medicine. 44 (2): 262–272. doi:10.1638/2012-0022R.1. ISSN 1042-7260. PMID 23805543.
- Snively, E.; Cotton, J. R.; Ridgely, R.; Witmer, L. M. (2013). "Multibody dynamics model of head and neck function in Allosaurus (Dinosauria, Theropoda)". Palaeontologia Electronica. 16 (2). doi:10.26879/338.
- "Dino Gangs". Discovery Channel. June 22, 2011. Archived from the original on January 19, 2012. Retrieved January 19, 2012.
- Collins, N. (June 22, 2011). "Tyrannosaurus Rex 'hunted in packs'". The Telegraph. Retrieved March 23, 2014.
- Wallis, P. (June 11, 2012). "Op-Ed: T. Rex pack hunters? Scary, but likely to be true". Digitaljournal.com. Retrieved December 23, 2015.
- Switek, B. (July 25, 2011). "A bunch of bones doesn't make a gang of bloodthirsty tyrannosaurs". The Guardian. Retrieved June 21, 2015.
- Sample, I. (July 23, 2014). "Researchers find first sign that tyrannosaurs hunted in packs". The Guardian. Retrieved July 28, 2014.
- McCrea, R. T. (2014). "A 'Terror of Tyrannosaurs': The First Trackways of Tyrannosaurids and Evidence of Gregariousness and Pathology in Tyrannosauridae". PLOS One. 9 (7): e103613. Bibcode:2014PLoSO...9j3613M. doi:10.1371/journal.pone.0103613. PMC 4108409. PMID 25054328.
- Peterson, J. E.; Henderson, M. D.; Sherer, R. P.; Vittore, C. P. (2009). "Face Biting On A Juvenile Tyrannosaurid And Behavioral Implications". PALAIOS. 24 (11): 780–784. Bibcode:2009Palai..24..780P. doi:10.2110/palo.2009.p09-056r. Archived from the original on August 11, 2013.
- Parisi, T. (November 2, 2009). "The terrible teens of T. rex NIU scientists: Young tyrannosaurs did serious battle against each other". Northern Illinois University. Archived from the original on August 14, 2013. Retrieved August 10, 2013.
- Rothschild, B.; Tanke, D. H.; Ford, T. L. (2001). "Theropod stress fractures and tendon avulsions as a clue to activity". In Tanke, D. H.; Carpenter, K. (eds.). Mesozoic Vertebrate Life. Indiana University Press. pp. 331–336.
- Wolff, E. D. S.; Salisbury, S. W.; Horner, J. R.; Varricchi, D. J. (2009). Hansen, D. M. (ed.). "Common Avian Infection Plagued the Tyrant Dinosaurs". PLOS One. 4 (9): e7288. Bibcode:2009PLoSO...4.7288W. doi:10.1371/journal.pone.0007288. PMC 2748709. PMID 19789646.
- Longrich, N. R.; Horner, J. R.; Erickson, G. M.; Currie, P. J. (2010). "Cannibalism in Tyrannosaurus rex". PLOS One. 5 (10): e13419. Bibcode:2010PLoSO...513419L. doi:10.1371/journal.pone.0013419. PMC 2955550. PMID 20976177.
- Perkins, S. (October 29, 2015). "Tyrannosaurs were probably cannibals (Comment)". Science. Retrieved November 2, 2015.
- Estes, R.; Berberian, P. (1970). "Paleoecology of a late Cretaceous vertebrate community from Montana". Breviora. 343: 1–35.
- Derstler, K. (1994). "Dinosaurs of the Lance Formation in eastern Wyoming". In Nelson, G. E. (ed.). The Dinosaurs of Wyoming. Wyoming Geological Association Guidebook, 44th Annual Field Conference. Wyoming Geological Association. pp. 127–146.
- Weishampel, D. B.; Barrett, P. M.; Coria, R. A.; Loeuff, J. L.; Xu, X.; Zhao, X.; Sahni, A.; Gomani, E. M. P.; Noto, C. R. (2004). "Dinosaur Distribution". In Weishampel, D. B.; Dodson, P.; Osmólska, H. (eds.). The Dinosauria (2nd ed.). Berkeley: University of California Press. pp. 574–588. ISBN 978-0-520-24209-8.
- Jasinski, S. E.; Sullivan, R. M.; Lucas, S. G. (2011). "Taxonomic composition of the Alamo Wash local fauna from the Upper Cretaceous Ojo Alamo Formation (Naashoibito Member) San Juan Basin, New Mexico". Bulletin. 53: 216–271.
- Serrano-Brañas, C. I.; Torres-Rodrígueza, E.; Luna, P. C. R.; González, I.; González-León, C. (2014). "Tyrannosaurid teeth from the Lomas Coloradas Formation, Cabullona Group (Upper Cretaceous) Sonora, México". Cretaceous Research. 49: 163–171. doi:10.1016/j.cretres.2014.02.018.
- Brusatte, S. L.; Carr, T. D. (2016). "The phylogeny and evolutionary history of tyrannosauroid dinosaurs". Scientific Reports. 6: 20252. Bibcode:2016NatSR...620252B. doi:10.1038/srep20252. PMC 4735739. PMID 26830019.
Further reading
- Farlow, J. O.; Gatesy, S. M.; Holtz, T. R., Jr.; Hutchinson, J. R.; Robinson, J. M. (2000). "Theropod Locomotion". American Zoologist. 40 (4): 640–663. doi:10.1093/icb/40.4.640. JSTOR 3884284.
External links
- The University of Edinburgh Lecture Dr Stephen Brusatte - Tyrannosaur Discoveries Feb 20, 2015
- 28 species in the tyrannosaur family tree, when and where they lived Stephen Brusatte Thomas Carr 2016
- Exhibits




.jpg)
.png)