Coral
Corals are marine invertebrates within the class Anthozoa of the phylum Cnidaria. They typically live in compact colonies of many identical individual polyps. Corals species include the important reef builders that inhabit tropical oceans and secrete calcium carbonate to form a hard skeleton.

A coral "group" is a colony of myriad genetically identical polyps. Each polyp is a sac-like animal typically only a few millimeters in diameter and a few centimeters in length. A set of tentacles surround a central mouth opening. Each polyp excretes an exoskeleton near the base. Over many generations, the colony thus creates a skeleton characteristic of the species which can measure up to several meters in size. Individual colonies grow by asexual reproduction of polyps. Corals also breed sexually by spawning: polyps of the same species release gametes simultaneously overnight, often around a full moon. Fertilized eggs form planulae, a mobile early form of the coral polyp which when mature settles to form a new colony.
Although some corals are able to catch plankton and small fish using stinging cells on their tentacles, most corals obtain the majority of their energy and nutrients from photosynthetic unicellular dinoflagellates of the genus Symbiodinium that live within their tissues. These are commonly known as zooxanthellae and gives the coral color. Such corals require sunlight and grow in clear, shallow water, typically at depths less than 60 metres (200 ft). Corals are major contributors to the physical structure of the coral reefs that develop in tropical and subtropical waters, such as the Great Barrier Reef off the coast of Australia. These corals are increasingly at risk of bleaching events where polyps expel the zooxanthellae in response to stress such as high water temperature or toxins.
Other corals do not rely on zooxanthellae and can live globally in much deeper water, such as the cold-water genus Lophelia which can survive as deep as 3,300 metres (10,800 ft).[1] Some have been found as far north as the Darwin Mounds, northwest of Cape Wrath, Scotland, and others off the coast of Washington State and the Aleutian Islands.
Taxonomy
The classification of corals has been discussed for millenia, owing to having similarities to both plants and animals. Aristotle's pupil Theophrastus described the red coral, korallion, in his book on stones, implying it was a mineral, but he described it as a deep-sea plant in his Enquiries on Plants, where he also mentions large stony plants that reveal bright flowers when under water in the Gulf of Heroes.[2] Pliny the Elder stated boldly that several sea creatures including sea nettles and sponges "are neither animals nor plants, but are possessed of a third nature (tertius natura)".[3] Petrus Gyllius copied Pliny, introducing the term zoophyta for this third group in his 1535 book On the French and Latin Names of the Fishes of the Marseilles Region; it is popularly but wrongly supposed that Aristotle created the term.[3] Gyllius further noted, following Aristotle, how hard it was to define what was a plant and what was an animal.[3]
The Persian polymath Al-Biruni (d.1048) classified sponges and corals as animals, arguing that they respond to touch.[4] Nevertheless, people believed corals to be plants until the eighteenth century, when William Herschel used a microscope to establish that coral had the characteristic thin cell membranes of an animal.[5]
Presently, corals are classified as species of animals within the sub-classes Hexacorallia and Octocorallia of the class Anthozoa in the phylum Cnidaria.[6] Hexacorallia includes the stony corals and these groups have polyps that generally have a 6-fold symmetry. Octocorallia includes blue coral and soft corals and species of Octocorallia have polyps with an eightfold symmetry, each polyp having eight tentacles and eight mesenteries.
Anatomy
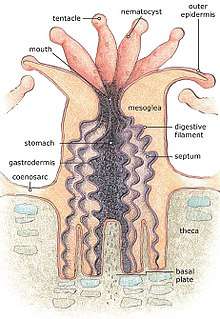
For most of their life corals are sessile animals of colonies of genetically identical polyps. Each polyp varies from millimeters to centimeters in diameter, and colonies can be formed from many million individual polyps. Stony coral, also known as hard coral, polyps produce a skeleton composed of calcium carbonate to strengthen and protect the organism. This is deposited by the polyps and by the coenosarc, the living tissue that connects them. The polyps sit in cup-shaped depressions in the skeleton known as corallites. Colonies of stony coral are very variable in appearance; a single species may adopt an encrusting, plate-like, bushy, columnar or massive solid structure, the various forms often being linked to different types of habitat, with variations in light level and water movement being significant.[7]
The body of the polyp may be roughly compared in a structure to a sac, the wall of which is composed of two layers of cells. The outer layer is known technically as the ectoderm, the inner layer as the endoderm. Between ectoderm and endoderm is a supporting layer of gelatinous substance termed mesoglea, secreted by the cell layers of the body wall.[8] The mesoglea can contain skeletal elements derived from cells migrated from ectoderm.
The sac-like body built up in this way is attached to a hard surface, which in hard corals are cup-shaped depressions in the skeleton known as corallites. At the center of the upper end of the sac lies the only opening called the mouth, surrounded by a circle of tentacles which resemble glove fingers. The tentacles are organs which serve both for the tactile sense and for the capture of food.[8] Polyps extend their tentacles, particularly at night, often containing coiled stinging cells (cnidocytes) which pierce, poison and firmly hold living prey paralysing or killing them. Polyp prey includes plankton such as copepods and fish larvae. Longitudinal muscular fibers formed from the cells of the ectoderm allow tentacles to contract to convey the food to the mouth. Similarly, circularly disposed muscular fibres formed from the endoderm permit tentacles to be protract or thrust out once they are contracted.[8] In both stony and soft corals, the polyps can be retracted by contracting muscle fibres, with stony corals relying on their hard skeleton and cnidocytes for defence. Soft corals generally secrete terpenoid toxins to ward off predators.[7]
In most corals, the tentacles are retracted by day and spread out at night to catch plankton and other small organisms. Shallow water species of both stony and soft corals can be zooxanthellate, the corals supplementing their plankton diet with the products of photosynthesis produced by these symbionts.[7] The polyps interconnect by a complex and well-developed system of gastrovascular canals, allowing significant sharing of nutrients and symbionts.[9]
The external form of the polyp varies greatly. The column may be long and slender, or may be so short in the vertical direction that the body becomes disk-like. The tentacles may number many hundreds or may be very few, in rare cases only one or two. They may be simple and unbranched, or feathery in pattern. The mouth may be level with the surface of the peristome, or may be projecting and trumpet-shaped.[8]
Soft corals
Soft corals have no solid exoskeleton as such. However, their tissues are often reinforced by small supportive elements known as "sclerites" made of calcium carbonate. The polyps of soft corals have eight-fold symmetry.
Soft corals vary considerably in form, and most are colonial. A few soft corals are stolonate, but the polyps of most are connected by sheets of tissue called coenosarc, and in some species these sheets are thick and the polyps deeply embedded in them. Some soft corals encrust other sea objects or form lobes. Others are tree-like or whip-like and chem a central axial skeleton embedded at its base in the matrix of the supporting branch.[10] These branches are composed either of a fibrous protein called gorgonin or of a calcified material.
Stony corals

The polyps of stony corals have six-fold symmetry. In stony corals the polyps are cylindrical and taper to a point, but in soft corals they are pinnate with side branches known as pinnules. In some tropical species these are reduced to mere stubs and in some they are fused to give a paddle-like appearance.[11]
Coral skeletons are biocomposites (mineral + organics) of calcium carbonate, in the form of calcite or aragonite. In scleractinian corals, "centers of calcification" and fibers are clearly distinct structures differing with respect to both morphology and chemical compositions of the crystalline units.[12][13] The organic matrices extracted from diverse species are acidic, and comprise proteins, sulphated sugars and lipids; they are species specific.[14] The soluble organic matrices of the skeletons allow to differentiate zooxanthellae and non-zooxanthellae specimens.[15]
Ecology
Feeding
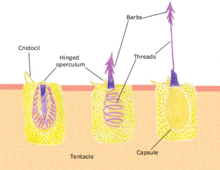
Polyps feed on a variety of small organisms, from microscopic zooplankton to small fish. The polyp's tentacles immobilize or kill prey using stinging cells called nematocysts. These cells carry venom which they rapidly release in response to contact with another organism. A dormant nematocyst discharges in response to nearby prey touching the trigger (Cnidocil). A flap (operculum) opens and its stinging apparatus fires the barb into the prey. The venom is injected through the hollow filament to immobilise the prey; the tentacles then manoeuvre the prey into the stomach. Once the prey is digested the stomach reopens allowing the elimination of waste products and the beginning of the next hunting cycle.[16]:24
Intracellular symbionts
Many corals, as well as other cnidarian groups such as sea anemones form a symbiotic relationship with a class of dinoflagellate algae, zooxanthellae of the genus Symbiodinium, which can form as much as 30% of the tissue of a polyp.[16]:23-24 Typically, each polyp harbors one species of alga, and coral species show a preference for Symbiodinium.[17] Young corals are not born with zooxanthellae, but acquire the algae from the surrounding environment, including the water column and local sediment.[18] The main benefit of the zooxanthellae is their ability to photosynthesize which supplies corals with the products of photosynthesis, including glucose, glycerol, and amino acids, which the corals can use for energy.[19] Zooxanthellae also benefit corals by aiding in calcification, for the coral skeleton, and waste removal.[20][21] In addition to the soft tissue, microbiomes are also found in the coral's mucus and (in stony corals) the skeleton, with the latter showing the greatest microbial richness.[22]
The zooxanthellae benefit from a safe place to live and consume the polyp's carbon dioxide, phosphate and nitrogenous waste. Due to rising ocean temperatures the strain on the coral, often drives them to eject the algae. Mass ejections are known as coral bleaching because the algae contribute to coral coloration; some colors, however, are due to host coral pigments, such as green fluorescent proteins (GFPs). Ejection increases the polyp's chance of surviving short-term stress and if the stress subsides they can regain algae, possibly of a different species, at a later time. If the stressful conditions persist, the polyp eventually dies.[23] Zooxanthellae are located within the coral cytoplasm and due to the algae's photosynthetic activity the internal pH of the coral can be raised; this behavior indicates that the zooxanthellae are responsible to some extent for the metabolism of their host corals [24]
Reproduction
Corals can be both gonochoristic (unisexual) and hermaphroditic, each of which can reproduce sexually and asexually. Reproduction also allows coral to settle in new areas. Reproduction is coordinated by chemical communication.
Sexual
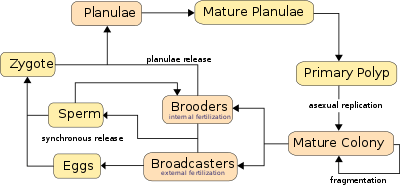
Corals predominantly reproduce sexually. About 25% of hermatypic corals (stony corals) form single sex (gonochoristic) colonies, while the rest are hermaphroditic.[25]
Broadcasters
About 75% of all hermatypic corals "broadcast spawn" by releasing gametes—eggs and sperm—into the water to spread offspring. The gametes fuse during fertilization to form a microscopic larva called a planula, typically pink and elliptical in shape. A typical coral colony forms several thousand larvae per year to overcome the odds against formation of a new colony.[26]

Synchronous spawning is very typical on the coral reef, and often, even when multiple species are present, all corals spawn on the same night. This synchrony is essential so male and female gametes can meet. Corals rely on environmental cues, varying from species to species, to determine the proper time to release gametes into the water. The cues involve temperature change, lunar cycle, day length, and possibly chemical signalling.[25] Synchronous spawning may form hybrids and is perhaps involved in coral speciation.[27] The immediate cue is most often sunset, which cues the release.[25] The spawning event can be visually dramatic, clouding the usually clear water with gametes.
Brooders
Brooding species are most often ahermatypic (not reef-building) in areas of high current or wave action. Brooders release only sperm, which is negatively buoyant, sinking on to the waiting egg carriers who harbor unfertilized eggs for weeks. Synchronous spawning events sometimes occur even with these species.[25] After fertilization, the corals release planula that are ready to settle.[20]
Planulae
Planula larvae exhibit positive phototaxis, swimming towards light to reach surface waters, where they drift and grow before descending to seek a hard surface to which they can attach and begin a new colony. They also exhibit positive sonotaxis, moving towards sounds that emanate from the reef and away from open water.[28] High failure rates afflict many stages of this process, and even though millions of gametes are released by each colony, few new colonies form. The time from spawning to settling is usually two to three days, but can be up to two months.[29] The larva grows into a polyp and eventually becomes a coral head by asexual budding and growth.
Asexual

Within a coral head, the genetically identical polyps reproduce asexually, either by budding (gemmation) or by dividing, whether longitudinally or transversely.
Budding involves splitting a smaller polyp from an adult.[26] As the new polyp grows, it forms its body parts. The distance between the new and adult polyps grows, and with it, the coenosarc (the common body of the colony). Budding can be intratentacular, from its oral discs, producing same-sized polyps within the ring of tentacles, or extratentacular, from its base, producing a smaller polyp.
Division forms two polyps that each become as large as the original. Longitudinal division begins when a polyp broadens and then divides its coelenteron (body), effectively splitting along its length. The mouth divides and new tentacles form. The two polyps thus created then generate their missing body parts and exoskeleton. Transversal division occurs when polyps and the exoskeleton divide transversally into two parts. This means one has the basal disc (bottom) and the other has the oral disc (top); the new polyps must separately generate the missing pieces.
Asexual reproduction offers the benefits of high reproductive rate, delaying senescence, and replacement of dead modules, as well as geographical distribution.[30]
Colony division
Whole colonies can reproduce asexually, forming two colonies with the same genotype. The possible mechanisms include fission, bailout and fragmentation. Fission occurs in some corals, especially among the family Fungiidae, where the colony splits into two or more colonies during early developmental stages. Bailout occurs when a single polyp abandons the colony and settles on a different substrate to create a new colony. Fragmentation involves individuals broken from the colony during storms or other disruptions. The separated individuals can start new colonies.[31]
Reefs
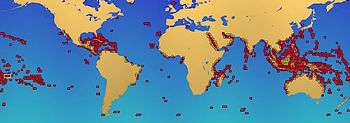
Many corals in the order Scleractinia are hermatypic, meaning that they are involved in building reefs. Most such corals obtain some of their energy from zooxanthellae in the genus Symbiodinium. These are symbiotic photosynthetic dinoflagellates which require sunlight; reef-forming corals are therefore found mainly in shallow water. They secrete calcium carbonate to form hard skeletons that become the framework of the reef. However, not all reef-building corals in shallow water contain zooxanthellae, and some deep water species, living at depths to which light cannot penetrate, form reefs but do not harbour the symbionts.[32]

There are various types of shallow-water coral reef, including fringing reefs, barrier reefs and atolls; most occur in tropical and subtropical seas. They are very slow-growing, adding perhaps one centimetre (0.4 in) in height each year. The Great Barrier Reef is thought to have been laid down about two million years ago. Over time, corals fragment and die, sand and rubble accumulates between the corals, and the shells of clams and other molluscs decay to form a gradually evolving calcium carbonate structure.[33] Coral reefs are extremely diverse marine ecosystems hosting over 4,000 species of fish, massive numbers of cnidarians, molluscs, crustaceans, and many other animals.[34]
Evolutionary history

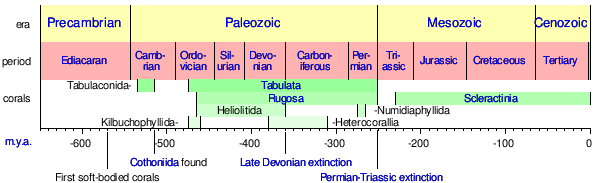
Although corals first appeared in the Cambrian period,[35] some 535 million years ago, fossils are extremely rare until the Ordovician period, 100 million years later, when rugose and tabulate corals became widespread. Paleozoic corals often contained numerous endobiotic symbionts.[36][37]


Tabulate corals occur in limestones and calcareous shales of the Ordovician and Silurian periods, and often form low cushions or branching masses of calcite alongside rugose corals. Their numbers began to decline during the middle of the Silurian period, and they became extinct at the end of the Permian period, 250 million years ago.[38]
Rugose or horn corals became dominant by the middle of the Silurian period, and became extinct early in the Triassic period. The rugose corals existed in solitary and colonial forms, and were also composed of calcite.[39]
The scleractinian corals filled the niche vacated by the extinct rugose and tabulate species. Their fossils may be found in small numbers in rocks from the Triassic period, and became common in the Jurassic and later periods.[40] Scleractinian skeletons are composed of a form of calcium carbonate known as aragonite.[41] Although they are geologically younger than the tabulate and rugose corals, the aragonite of their skeletons is less readily preserved, and their fossil record is accordingly less complete.
 | |
|
Timeline of the major coral fossil record and developments from 650 m.y.a. to present.[42][43] |
|
At certain times in the geological past, corals were very abundant. Like modern corals, these ancestors built reefs, some of which ended as great structures in sedimentary rocks. Fossils of fellow reef-dwellers algae, sponges, and the remains of many echinoids, brachiopods, bivalves, gastropods, and trilobites appear along with coral fossils. This makes some corals useful index fossils.[44] Coral fossils are not restricted to reef remnants, and many solitary fossils may be found elsewhere, such as Cyclocyathus, which occurs in England's Gault clay formation.
Status
Threats

Coral reefs are under stress around the world.[45] In particular, coral mining, agricultural and urban runoff, pollution (organic and inorganic), overfishing, blast fishing, disease, and the digging of canals and access into islands and bays are localized threats to coral ecosystems. Broader threats are sea temperature rise, sea level rise and pH changes from ocean acidification, all associated with greenhouse gas emissions.[46] In 1998, 16% of the world's reefs died as a result of increased water temperature.[47]
Approximately 10% of the world's coral reefs are dead.[48][49][50] About 60% of the world's reefs are at risk due to human-related activities.[51] The threat to reef health is particularly strong in Southeast Asia, where 80% of reefs are endangered.[52] Over 50% of the world's coral reefs may be destroyed by 2030; as a result, most nations protect them through environmental laws.[53]
In the Caribbean and tropical Pacific, direct contact between ~40–70% of common seaweeds and coral causes bleaching and death to the coral via transfer of lipid-soluble metabolites.[54] Seaweed and algae proliferate given adequate nutrients and limited grazing by herbivores such as parrotfish.
Water temperature changes of more than 1–2 °C (1.8–3.6 °F) or salinity changes can kill some species of coral. Under such environmental stresses, corals expel their Symbiodinium; without them coral tissues reveal the white of their skeletons, an event known as coral bleaching.[55]
Submarine springs found along the coast of Mexico's Yucatán Peninsula produce water with a naturally low pH (relatively high acidity) providing conditions similar to those expected to become widespread as the oceans absorb carbon dioxide.[56] Surveys discovered multiple species of live coral that appeared to tolerate the acidity. The colonies were small and patchily distributed, and had not formed structurally complex reefs such as those that compose the nearby Mesoamerican Barrier Reef System.[56]
Protection
Marine Protected Areas, Biosphere reserves, marine parks, national monuments world heritage status, fishery management and habitat protection can protect reefs from anthropogenic damage.[57]
Many governments now prohibit removal of coral from reefs, and inform coastal residents about reef protection and ecology. While local action such as habitat restoration and herbivore protection can reduce local damage, the longer-term threats of acidification, temperature change and sea-level rise remain a challenge.[46]
To eliminate destruction of corals in their indigenous regions, projects have been started to grow corals in non-tropical countries.[58][59]
Relation to humans
Local economies near major coral reefs benefit from an abundance of fish and other marine creatures as a food source. Reefs also provide recreational scuba diving and snorkeling tourism. These activities can damage coral but international projects such as Green Fins that encourage dive and snorkel centres to follow a Code of Conduct have been proven to mitigate these risks.[60]
Jewelry
%2C_ca._1920s%2C_cropped.jpg)
Corals' many colors give it appeal for necklaces and other jewelry. Intensely red coral is prized as a gemstone. Sometimes called fire coral, it is not the same as fire coral. Red coral is very rare because of overharvesting.[61] In general, it is inadvisable to give coral as gifts since they are in decline from stressors like climate change, pollution, and unsustainable fishing.
Always considered a precious mineral, "the Chinese have long associated red coral with auspiciousness and longevity because of its color and its resemblance to deer antlers (so by association, virtue, long life, and high rank".[62] It reached its height of popularity during the Manchu or Qing Dynasty (1644-1911) when it was almost exclusively reserved for the emperor's use either in the form of coral beads (often combined with pearls) for court jewelry or as decorative Penjing (decorative miniature mineral trees). Coral was known as shanhu in Chinese. The "early-modern 'coral network' [began in] the Mediterranean Sea [and found its way] to Qing China via the English East India Company".[63] There were strict rules regarding its use in a code established by the Qianlong Emperor in 1759.
Medicine

In medicine, chemical compounds from corals can potentially be used to treat cancer, AIDS, pain, and for other therapeutic uses.[65][66] Coral skeletons, e.g. Isididae are also used for bone grafting in humans.[67] Coral Calx, known as Praval Bhasma in Sanskrit, is widely used in traditional system of Indian medicine as a supplement in the treatment of a variety of bone metabolic disorders associated with calcium deficiency.[68] In classical times ingestion of pulverized coral, which consists mainly of the weak base calcium carbonate, was recommended for calming stomach ulcers by Galen and Dioscorides.[69]
Construction
Coral reefs in places such as the East African coast are used as a source of building material.[70] Ancient (fossil) coral limestone, notably including the Coral Rag Formation of the hills around Oxford (England), was once used as a building stone, and can be seen in some of the oldest buildings in that city including the Saxon tower of St Michael at the Northgate, St. George's Tower of Oxford Castle, and the medieval walls of the city.[71]
Shoreline protection
Healthy coral reefs absorb 97 percent of a wave’s energy, which buffers shorelines from currents, waves, and storms, helping to prevent loss of life and property damage. Coastlines protected by coral reefs are also more stable in terms of erosion than those without.[72]
Local economies
Coastal communities near coral reefs rely heavily on them. Worldwide, more than 500 million people depend on coral reefs for food, income, coastal protection, and more.[73] The total economic value of coral reef services in the United States - including fisheries, tourism, and coastal protection - is more than $3.4 billion a year.
Climate research
Annual growth bands in some corals, such as the deep sea bamboo corals (Isididae), may be among the first signs of the effects of ocean acidification on marine life.[74] The growth rings allow geologists to construct year-by-year chronologies, a form of incremental dating, which underlie high-resolution records of past climatic and environmental changes using geochemical techniques.[75]
Certain species form communities called microatolls, which are colonies whose top is dead and mostly above the water line, but whose perimeter is mostly submerged and alive. Average tide level limits their height. By analyzing the various growth morphologies, microatolls offer a low resolution record of sea level change. Fossilized microatolls can also be dated using Radiocarbon dating. Such methods can help to reconstruct Holocene sea levels.[76]
Increasing sea temperatures in tropical regions (~1 degree C) the last century have caused major coral bleaching, death, and therefore shrinking coral populations since although they are able to adapt and acclimate, it is uncertain if this evolutionary process will happen quickly enough to prevent major reduction of their numbers.[77]
Though coral have large sexually-reproducing populations, their evolution can be slowed by abundant asexual reproduction.[78] Gene flow is variable among coral species.[78] According to the biogeography of coral species gene flow cannot be counted on as a dependable source of adaptation as they are very stationary organisms. Also, coral longevity might factor into their adaptivity.[78]
However, adaptation to climate change has been demonstrated in many cases. These are usually due to a shift in coral and zooxanthellae genotypes. These shifts in allele frequency have progressed toward more tolerant types of zooxanthellae.[79] Scientists found that a certain scleractinian zooxanthella is becoming more common where sea temperature is high.[80][81] Symbionts able to tolerate warmer water seem to photosynthesise more slowly, implying an evolutionary trade-off.[81]
In the Gulf of Mexico, where sea temperatures are rising, cold-sensitive staghorn and elkhorn coral have shifted in location.[79] Not only have the symbionts and specific species been shown to shift, but there seems to be a certain growth rate favorable to selection. Slower-growing but more heat-tolerant corals have become more common.[82] The changes in temperature and acclimation are complex. Some reefs in current shadows represent a refugium location that will help them adjust to the disparity in the environment even if eventually the temperatures may rise more quickly there than in other locations.[83] This separation of populations by climatic barriers causes a realized niche to shrink greatly in comparison to the old fundamental niche.
Geochemistry
Corals are shallow, colonial organisms that integrate oxygen and trace elements into their skeletal aragonite (polymorph of calcite) crystalline structures as they grow. Geochemical anomalies within the crystalline structures of corals represent functions of temperature, salinity and oxygen isotopic composition. Such geochemical analysis can help with climate modeling.[84] The ratio of oxygen-18 to oxygen-16 (δ18O), for example, is a proxy for temperature.
Strontium/calcium ratio anomaly
Time can be attributed to coral geochemistry anomalies by correlating strontium/calcium minimums with sea surface temperature (SST) maximums to data collected from NINO 3.4 SSTA.[85]
Oxygen isotope anomaly
The comparison of coral strontium/calcium minimums with sea surface temperature maximums, data recorded from NINO 3.4 SSTA, time can be correlated to coral strontium/calcium and δ18O variations. To confirm accuracy of the annual relationship between Sr/Ca and δ18O variations, a perceptible association to annual coral growth rings confirms the age conversion. Geochronology is established by the blending of Sr/Ca data, growth rings, and stable isotope data. El Nino-Southern Oscillation (ENSO) is directly related to climate fluctuations that influence coral δ18O ratio from local salinity variations associated with the position of the South Pacific convergence zone (SPCZ) and can be used for ENSO modeling.[85]
Sea surface temperature and sea surface salinity
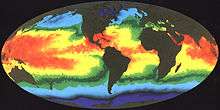
The global moisture budget is primarily being influenced by tropical sea surface temperatures from the position of the Intertropical Convergence Zone (ITCZ).[86] The Southern Hemisphere has a unique meteorological feature positioned in the southwestern Pacific Basin called the South Pacific Convergence Zone (SPCZ), which contains a perennial position within the Southern Hemisphere. During ENSO warm periods, the SPCZ reverses orientation extending from the equator down south through Solomon Islands, Vanuatu, Fiji and towards the French Polynesian Islands; and due east towards South America affecting geochemistry of corals in tropical regions.[87]
Geochemical analysis of skeletal coral can be linked to sea surface salinity (SSS) and sea surface temperature (SST), from El Nino 3.4 SSTA data, of tropical oceans to seawater δ18O ratio anomalies from corals. ENSO phenomenon can be related to variations in sea surface salinity (SSS) and sea surface temperature (SST) that can help model tropical climate activities.[88]
Limited climate research on current species

Climate research on live coral species is limited to a few studied species. Studying Porites coral provides a stable foundation for geochemical interpretations that is much simpler to physically extract data in comparison to Platygyra species where the complexity of Platygyra species skeletal structure creates difficulty when physically sampled, which happens to be one of the only multidecadal living coral records used for coral paleoclimate modeling.[88]
Aquaria

The saltwater fishkeeping hobby has expanded, over recent years, to include reef tanks, fish tanks that include large amounts of live rock on which coral is allowed to grow and spread.[89] These tanks are either kept in a natural-like state, with algae (sometimes in the form of an algae scrubber) and a deep sand bed providing filtration,[90] or as "show tanks", with the rock kept largely bare of the algae and microfauna that would normally populate it,[91] in order to appear neat and clean.
The most popular kind of coral kept is soft coral, especially zoanthids and mushroom corals, which are especially easy to grow and propagate in a wide variety of conditions, because they originate in enclosed parts of reefs where water conditions vary and lighting may be less reliable and direct.[92] More serious fishkeepers may keep small polyp stony coral, which is from open, brightly lit reef conditions and therefore much more demanding, while large polyp stony coral is a sort of compromise between the two.
Aquaculture
Coral aquaculture, also known as coral farming or coral gardening, is the cultivation of corals for commercial purposes or coral reef restoration. Aquaculture is showing promise as a potentially effective tool for restoring coral reefs, which have been declining around the world.[93][94][95] The process bypasses the early growth stages of corals when they are most at risk of dying. Coral fragments known as "seeds" are grown in nurseries then replanted on the reef.[96] Coral is farmed by coral farmers who live locally to the reefs and farm for reef conservation or for income. It is also farmed by scientists for research, by businesses for the supply of the live and ornamental coral trade and by private aquarium hobbyists.
Gallery
Further images: commons:Category:Coral reefs and commons:Category:Corals
- Fungia sp. skeleton
 Polyps of Eusmilia fastigiata
Polyps of Eusmilia fastigiata_(San_Salvador_Island%2C_Bahamas)_1_(15513345363).jpg) Pillar coral, Dendrogyra cylindricus
Pillar coral, Dendrogyra cylindricus Brain coral, Diploria labyrinthiformis
Brain coral, Diploria labyrinthiformis Brain coral spawning
Brain coral spawning Brain coral releasing eggs
Brain coral releasing eggs
References
- Squires, D.F. (1959). "Deep sea corals collected by the Lamont Geological Observatory. 1. Atlantic corals" (PDF). American Museum Novitates. 1965: 23.
- Leroi, Armand Marie (2014). The Lagoon: How Aristotle Invented Science. Bloomsbury. p. 271. ISBN 978-1-4088-3622-4.
- Bowen, James (2015). The Coral Reef Era: From Discovery to Decline: A history of scientific investigation from 1600 to the Anthropocene Epoch. Springer. pp. 5–7. ISBN 978-3-319-07479-5.
- Egerton, Frank N. (2012). Roots of Ecology: Antiquity to Haeckel. University of California Press. p. 24. ISBN 978-0-520-95363-5.
- Swett, C. Corals: Secrets of Their Reef-Making Colonies. Capstone Global Library Ltd. ISBN 9781474771009.
- Hoeksema, Bert (2015). "Anthozoa". WoRMS. World Register of Marine Species. Retrieved 2015-04-24.
- Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology, 7th edition. Cengage Learning. pp. 132–48. ISBN 978-81-315-0104-7.
-

- D. Gateno; A. Israel; Y. Barki; B. Rinkevich (1998). "Gastrovascular Circulation in an Octocoral: Evidence of Significant Transport of Coral and Symbiont Cells". The Biological Bulletin. 194 (2): 178–86. doi:10.2307/1543048. JSTOR 1543048. PMID 28570841.
- Administration, US Department of Commerce, National Oceanic and Atmospheric. "existing and potential value of coral ecosystems with respect to income and other economic values". coralreef.noaa.gov. Retrieved 2018-02-04.
- Sprung, Julian (1999). Corals: A quick reference guide. Ricordea Publishing. p. 145. ISBN 978-1-883693-09-1.
- Cuif, J.P.; Dauphin, Y. (1998). "Microstructural and physico-chemical characterization of 'centers of calcification' in septa of some Recent scleractinian corals". Paläontologische Zeitschrift. 72 (3–4): 257–269. doi:10.1007/bf02988357. ISSN 0031-0220.
- Cuif, J.P.; Dauphin, Y.; Doucet, J.; Salomé, M.; Susini, J. (2003). "XANES mapping of organic sulfate in three scleractinian coral skeletons". Geochimica et Cosmochimica Acta. 67 (1): 75–83. doi:10.1016/s0016-7037(02)01041-4. ISSN 0016-7037.
- Dauphin, Y.; Cuif, J.P.; Williams, C. T. (2008). "Soluble organic matrices of aragonitic skeletons of Merulinidae (Cnidaria, Anthozoa)". Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 150 (1): 10–22. doi:10.1016/j.cbpb.2008.01.002. ISSN 1096-4959. PMID 18325807.
- Cuif, J.P.; Dauphin, Y.; Freiwald, A.; Gautret, P.; Zibrowius, H. (1999). "Biochemical markers of zooxanthellae symbiosis in soluble matrices of skeleton of 24 Scleractinia species". Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 123 (3): 269–278. doi:10.1016/s1095-6433(99)00059-8. ISSN 1095-6433.
- Murphy, Richard C. (2002). Coral Reefs: Cities Under The Seas. The Darwin Press. ISBN 978-0-87850-138-0.
- Yuyama, Ikuko (2014). "Comparing the Effects of Symbiotic Algae (Symbiodinium) Clades C1 and D on Early Growth Stages of Acropora tenuis". PLOS ONE. 9 (6): e98999. Bibcode:2014PLoSO...998999Y. doi:10.1371/journal.pone.0098999. PMC 4051649. PMID 24914677.
- Yamashita, Hiroshi (2014). "Establishment of Coral–Algal Symbiosis Requires Attraction and Selection". PLOS ONE. 9 (5): e97003. Bibcode:2014PLoSO...997003Y. doi:10.1371/journal.pone.0097003. PMC 4019531. PMID 24824794.
- "Zooxanthellae...What's That?". NOAA Ocean Service Education. National Oceanic and Atmospheric Administration. Retrieved 1 December 2017.
- Madl, P.; Yip, M. (2000). "Field Excursion to Milne Bay Province – Papua New Guinea". Retrieved 2006-03-31.
- van de Plaasche, Orson (1986). Sea-level research: a manual for the collection and evaluation of data. Norwich, UK: Geo Books. p. 196. ISBN 978-94-010-8370-6.
- Corals and their microbiomes evolved together | Penn State University
- W. W. Toller; R. Rowan; N. Knowlton (2001). "Repopulation of Zooxanthellae in the Caribbean Corals Montastraea annularis and M. faveolata following Experimental and Disease-Associated Bleaching". The Biological Bulletin. 201 (3): 360–73. doi:10.2307/1543614. JSTOR 1543614. PMID 11751248.
- Brownlee, Colin (2009). "pH regulation in symbiotic anemones and corals: A delicate balancing act". Proceedings of the National Academy of Sciences of the United States of America. 106 (39): 16541–16542. Bibcode:2009PNAS..10616541B. doi:10.1073/pnas.0909140106. PMC 2757837. PMID 19805333.
- Veron, J.E.N. (2000). Corals of the World. Vol 3 (3rd ed.). Australia: Australian Institute of Marine Sciences and CRR Qld. ISBN 978-0-642-32236-4.
- Barnes, R. and; Hughes, R. (1999). An Introduction to Marine Ecology (3rd ed.). Malden, MA: Blackwell. pp. 117–41. ISBN 978-0-86542-834-8.
- Hatta, M.; Fukami, H.; Wang, W.; Omori, M.; Shimoike, K.; Hayashibara, T.; Ina, Y.; Sugiyama, T. (1999). "Reproductive and genetic evidence for a reticulate evolutionary theory of mass spawning corals" (PDF). Molecular Biology and Evolution. 16 (11): 1607–13. doi:10.1093/oxfordjournals.molbev.a026073. PMID 10555292.
- Vermeij, Mark J. A.; Marhaver, Kristen L.; Huijbers, Chantal M.; Nagelkerken, Ivan; Simpson, Stephen D. (2010). "Coral Larvae Move toward Reef Sounds". PLoS ONE. 5 (5): e10660. Bibcode:2010PLoSO...510660V. doi:10.1371/journal.pone.0010660. PMC 2871043. PMID 20498831. Lay summary – ScienceDaily (May 16, 2010).
- Jones, O.A.; Endean, R. (1973). Biology and Geology of Coral Reefs. New York, USA: Harcourt Brace Jovanovich. pp. 205–45. ISBN 978-0-12-389602-5.
- Gulko, David (1998). Hawaiian Coral Reef Ecology. Honolulu, Hawaii: Mutual Publishing. p. 10. ISBN 978-1-56647-221-0.
- Sheppard, Charles R.C.; Davy, Simon K.; Pilling, Graham M. (25 June 2009). The Biology of Coral Reefs. OUP Oxford. pp. 78–81. ISBN 978-0-19-105734-2.
- Schuhmacher, Helmut; Zibrowius, Helmut (1985). "What is hermatypic?". Coral Reefs. 4 (1): 1–9. Bibcode:1985CorRe...4....1S. doi:10.1007/BF00302198.
- MSN Encarta (2006). Great Barrier Reef. Archived from the original on October 28, 2009. Retrieved April 25, 2015.
- Spalding, Mark; Ravilious, Corinna; Green, Edmund (2001). World Atlas of Coral Reefs. Berkeley, CA: University of California Press and UNEP/WCMC. pp. 205–45. ISBN 978-0-520-23255-6.
- Pratt, B.R.; Spincer, B.R.; Wood, R.A.; Zhuravlev, A.Yu. (2001). "12: Ecology and Evolution of Cambrian Reefs" (PDF). Ecology of the Cambrian Radiation. Columbia University Press. p. 259. ISBN 978-0-231-10613-9. Retrieved 2007-04-06.
- Vinn, O.; Mõtus, M.-A. (2008). "The earliest endosymbiotic mineralized tubeworms from the Silurian of Podolia, Ukraine". Journal of Paleontology. 82 (2): 409–14. doi:10.1666/07-056.1. Retrieved 2014-06-11.
- Vinn, O.; Mõtus, M.-A. (2012). "Diverse early endobiotic coral symbiont assemblage from the Katian (Late Ordovician) of Baltica". Palaeogeography, Palaeoclimatology, Palaeoecology. 321–322: 137–41. doi:10.1016/j.palaeo.2012.01.028.
- "Introduction to the Tabulata". UCMP Berkeley. Archived from the original on 19 April 2015. Retrieved 25 April 2015.
- "Introduction to the Rugosa". UCMP Berkeley. Archived from the original on 19 April 2015. Retrieved 25 April 2015.
- "Evolutionary history". AIMS. Retrieved 25 April 2015.
- Ries JB, Stanley SM, Hardie LA (July 2006). "Scleractinian corals produce calcite, and grow more slowly, in artificial Cretaceous seawater". Geology. 34 (7): 525–28. Bibcode:2006Geo....34..525R. doi:10.1130/G22600.1.
- Waggoner, Ben M. (2000). Smith, David; Collins, Allen (eds.). "Anthozoa: Fossil Record". Anthozoa. UCMP. Retrieved 9 March 2020.
- Oliver, William A. Jr. (2003). "Corals: Table 1". Fossil Groups. USGS. Archived from the original on 9 January 2009. Retrieved 9 March 2020.
- Alden, Andrew. "Index Fossils". About education. Retrieved 25 April 2015.
- "Coral reefs around the world". Guardian.co.uk. 2 September 2009.
- "Threats to Coral Reefs". Coral Reef Alliance. 2010. Archived from the original on 1 December 2011. Retrieved 5 December 2011.
- Losing Our Coral Reefs – Eco Matters – State of the Planet. Blogs.ei.columbia.edu. Retrieved on 2011-11-01.
- Kleypas, J.A.; Feely, R.A.; Fabry, V.J.; Langdon, C.; Sabine, C.L.; Robbins, L.L. (2006). "Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers: A guide for Future Research" (PDF). National Science Foundation, NOAA, & United States Geological Survey. Archived from the original (PDF) on July 20, 2011. Retrieved April 7, 2011. Cite journal requires
|journal=(help) - Save Our Seas, 1997 Summer Newsletter, Dr. Cindy Hunter and Dr. Alan Friedlander
- Tun, K.; Chou, L.M.; Cabanban, A.; Tuan, V.S.; Philreefs, S.; Yeemin, T.; Suharsono; Sour, K.; Lane, D. (2004). "Status of Coral Reefs, Coral Reef Monitoring and Management in Southeast Asia, 2004". In Wilkinson, C. (ed.). Status of Coral Reefs of the world: 2004. Townsville, Queensland, Australia: Australian Institute of Marine Science. pp. 235–76. Retrieved 2019-04-23.
- Burke, Lauretta; Reytar, K.; Spalding, M.; Perry, A. (2011). Reefs at risk revisited. Washington, DC: World Resources Institute. p. 38. ISBN 978-1-56973-762-0.
- Bryant, Dirk; Burke, Lauretta; McManus, John; Spalding, Mark. "Reefs at Risk: A Map-Based Indicator of Threats to the World's Coral Reef" (PDF). NOAA. Archived from the original (PDF) on 2013-02-18. Retrieved 25 April 2015.
- Norlander (8 December 2003). "Coral crisis! Humans are killing off these bustling underwater cities. Can coral reefs be saved? (Life science: corals)". Science World.
- Rasher DB, Hay ME (May 2010). "Chemically rich seaweeds poison corals when not controlled by herbivores". Proceedings of the National Academy of Sciences of the United States of America. 107 (21): 9683–88. Bibcode:2010PNAS..107.9683R. doi:10.1073/pnas.0912095107. PMC 2906836. PMID 20457927.
- Hoegh-Guldberg, O. (1999). "Climate change, coral bleaching and the future of the world's coral reefs" (PDF). Marine and Freshwater Research. 50 (8): 839–66. doi:10.1071/MF99078. Archived from the original (PDF) on 2012-04-26.
- Stephens, Tim (28 November 2011). "Submarine springs offer preview of ocean acidification effects on coral reefs". University of California Santa Cruz. Retrieved 25 April 2015.
- "Phoenix Rising". National Geographic Magazine. January 2011. Retrieved April 30, 2011.
- EcoDeco EcologicalTechnology Archived 2011-03-07 at the Wayback Machine. Ecodeco.nl. Retrieved on 2011-11-29.
- KoralenKAS project Archived 2012-04-26 at the Wayback Machine. Koraalwetenschap.nl. Retrieved on 2011-11-29.
- Hunt, Chloe V.; Harvey, James J.; Miller, Anne; Johnson, Vivienne; Phongsuwan, Niphon (2013). "The Green Fins approach for monitoring and promoting environmentally sustainable scuba diving operations in South East Asia". Ocean & Coastal Management. 78: 35–44. doi:10.1016/j.ocecoaman.2013.03.004.
- Magsaysay, Melissa (June 21, 2009). "Coral makes a splash". Los Angeles Times. Retrieved January 12, 2013.
- Welch, Patricia Bjaaland, Chinese Art: A Guide to Motifs and Visual Imagery. Tokyo, Rutland and Singapore: Tuttle, 2008, p. 61
- Lacey, Pippa, "The Coral Network: The trade of red coral to the Qing imperial court in the eighteenth century" in The Global Lives of Things, ed. by Anne Gerritsen and Giorgio Aiello, London: Rutledge, 2016, p. 81
- Folio 391, Juliana Anicia Codex
- Copper, Edwin; Hirabayashi, K.; Strychar, K. B.; Sammarco, P. W. (2014). "Corals and their Potential Applications to Integrative Medicine". Evidence-Based Complementary and Alternative Medicine. 2014: 9. doi:10.1155/2014/184959. PMC 3976867. PMID 24757491.
- Senthilkumar, Kalimuthu; Se-Kwon, Kim (2013). "Marine Invertebrate Natural Products for Anti-Inflammatory and Chronic Diseases". Evidence-Based Complementary and Alternative Medicine. 2013: 572859. doi:10.1155/2013/572859. PMC 3893779. PMID 24489586.
- Ehrlich, H.; Etnoyer, P.; Litvinov, S. D.; Olennikova, M.M.; Domaschke, H.; Hanke, T.; Born, R.; Meissner, H.; Worch, H. (2006). "Biomaterial structure in deep‐sea bamboo coral (Anthozoa: Gorgonacea: Isididae): perspectives for the development of bone implants and templates for tissue engineering". Materialwissenschaft und Werkstofftechnik. 37 (6): 552–57. doi:10.1002/mawe.200600036.
- Reddy PN, Lakshmana M, Udupa UV (December 2003). "Effect of Praval bhasma (Coral calx), a natural source of rich calcium on bone mineralization in rats". Pharmacological Research. 48 (6): 593–99. doi:10.1016/S1043-6618(03)00224-X. PMID 14527824.
- Pedanius Dioscorides – Der Wiener Dioskurides, Codex medicus Graecus 1 der Österreichischen Nationalbibliothek Graz: Akademische Druck- und Verlagsanstalt 1998 fol. 391 verso (Band 2), Kommentar S. 47 und 52. ISBN 3-201-01725-6
- Pouwels, Randall L. (6 June 2002). Horn and Crescent: Cultural Change and Traditional Islam on the East African Coast, 800–1900. Cambridge University Press. p. 26. ISBN 978-0-521-52309-7.
- "Strategic Stone Study: A Building Stone Atlas of Oxfordshire". English Heritage. March 2011. Retrieved 23 April 2015.
- Ferrario, F.; Beck, M.W.; Storlazzi, C.D.; Micheli, F.; Shepard, C.C.; Airoldi, L. (2014). "The effectiveness of coral reefs for coastal hazard risk reduction and adaptation". Nature Communications. 5 (3794): 3794. doi:10.1038/ncomms4794. PMC 4354160. PMID 24825660.
- "Status of Coral Reefs of the World: 2004 Volume 1" (PDF). Global Coral Reef Monitoring Network. Retrieved 2019-01-14.
- "National Oceanic and Atmospheric Administration – New Deep-Sea Coral Discovered on NOAA-Supported Mission". www.noaanews.noaa.gov. Retrieved 2009-05-11.
- Schrag, D.P.; Linsley, B.K. (2002). "Corals, chemistry, and climate". Science. 296 (8): 277–78. doi:10.1126/science.1071561. PMID 11951026.
- Smithers, Scott G.; Woodroffe, Colin D. (2000). "Microatolls as sea-level indicators on a mid-ocean atoll". Marine Geology. 168 (1–4): 61–78. Bibcode:2000MGeol.168...61S. doi:10.1016/S0025-3227(00)00043-8.
- Hoegh-Guldberg O. (1999). "Climate change, coral bleaching and the future of the world's coral reefs". Marine and Freshwater Research. 50 (8): 839–99. doi:10.1071/mf99078.
- Hughes, T.; Baird, A.; Bellwood, D.; Card, M.; Connolly, S.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.; Klepas, J.; Lough, J.; Marshall, P.; Nystrom, M.; Palumbi, S.; Pandolfi, J.; Rosen, B.; and Roughgarden, J. (2003). "Climate change, human impacts, and the resilience of coral reefs". Science. 301 (5635): 929–33. Bibcode:2003Sci...301..929H. doi:10.1126/science.1085046. PMID 12920289.
- Parmesan, C. (2006). "Ecological and evolutionary responses to recent climate change". Annual Review of Ecology, Evolution, and Systematics. 37: 637–69. doi:10.1146/annurev.ecolsys.37.091305.110100.
- Baker, A. (2004). "Corals' adaptive response to climate change". Nature. 430 (7001): 741. Bibcode:2004Natur.430..741B. doi:10.1038/430741a.
- Donner, S.; Skirving, W.; Little, C.; Oppenheimer, M.; Hoegh-Guldberg, O. (2005). "Global assessment of coral bleaching and required rates of adaptation under climate change" (PDF). Global Change Biology. 11 (12): 2251–65. Bibcode:2005GCBio..11.2251D. CiteSeerX 10.1.1.323.8134. doi:10.1111/j.1365-2486.2005.01073.x.
- Baskett, M.; Gaines, S. & Nisbet, R. (2009). "Symbiont diversity may help coral reefs survive moderate climate change" (PDF). Ecological Applications. 19 (1): 3–17. doi:10.1890/08-0139.1. PMID 19323170.
- McClanahan, T.; Ateweberhan, M.; Muhando, C.; Maina, J. & Mohammed, M. (2007). "Effects of Climate and Seawater Temperature Variation on Coral Bleaching and Morality". Ecological Monographs. 77 (4): 503–25. CiteSeerX 10.1.1.538.970. doi:10.1890/06-1182.1.
- Kilbourne, K. Halimeda; Quinn, Terrence M.; Taylor, Frederick W.; Delcroix, Thierry; Gouriou, Yves (2004). "El Niño-Southern Oscillation-related salinity variations recorded in the skeletal geochemistry of a Porites coral from Espiritu Santo, Vanuatu". Paleoceanography. 19 (4): PA4002. Bibcode:2004PalOc..19.4002K. doi:10.1029/2004PA001033.
- Ren, Lei; Linsley, Braddock K.; Wellington, Gerard M.; Schrag, Daniel P.; Hoegh-guldberg, Ove (2003). "Deconvolving the δ18O seawater component from subseasonal coral δ18O and Sr/Ca at Rarotonga in the southwestern subtropical Pacific for the period 1726 to 1997". Geochimica et Cosmochimica Acta. 67 (9): 1609–21. Bibcode:2003GeCoA..67.1609R. doi:10.1016/S0016-7037(02)00917-1.
- Wu, Henry C.; Linsley, Braddock K.; Dassié, Emilie P.; Schiraldi, Benedetto; deMenocal, Peter B. (2013). "Oceanographic variability in the South Pacific Convergence Zone region over the last 210 years from multi-site coral Sr/Ca records". Geochemistry, Geophysics, Geosystems. 14 (5): 1435–53. Bibcode:2013GGG....14.1435W. doi:10.1029/2012GC004293.
- Kiladis, George N.; von Storch, Hans; van Loon, Harry (1989). "Origin of the South Pacific Convergence Zone". Journal of Climate. 2 (10): 1185–95. doi:10.1175/1520-0442(1989)002<1185:OOTSPC>2.0.CO;2.
- Lukas, Roger; Lindstrom, Eric (1991). "The mixed layer of the western equatorial Pacific Ocean". Journal of Geophysical Research. 96 (S1): 3343–58. Bibcode:1991JGR....96.3343L. doi:10.1029/90JC01951.
- Aquarium Corals: Collection and Aquarium Husbandry of Northeast Pacific Non-Photosynthetic Cnidaria. Advancedaquarist.com (2011-01-14). Retrieved on 2016-06-13.
- Reefkeeping 101 – Various Nutrient Control Methods. Reefkeeping.com. Retrieved on 2016-06-13.
- Aquarium Substrate & Live Rock Clean Up Tips. Saltaquarium.about.com. Retrieved on 2016-06-13.
- Coral Reefs Archived 2013-01-21 at the Wayback Machine. Marinebio.org. Retrieved on 2016-06-13.
- Horoszowski-Fridman YB, Izhaki I, Rinkevich B (2011). "Engineering of coral reef larval supply through transplantation of nursery-farmed gravid colonies". Journal of Experimental Marine Biology and Ecology. 399 (2): 162–66. doi:10.1016/j.jembe.2011.01.005.
- Pomeroy, Robert S.; Parks, John E.; Balboa, Cristina M. (2006). "Farming the reef: Is aquaculture a solution for reducing fishing pressure on coral reefs?". Marine Policy. 30 (2): 111–30. doi:10.1016/j.marpol.2004.09.001.
- Rinkevich B (2008). "Management of coral reefs: We have gone wrong when neglecting active reef restoration" (PDF). Marine Pollution Bulletin. 56 (11): 1821–24. doi:10.1016/j.marpolbul.2008.08.014. PMID 18829052. Archived from the original (PDF) on 2013-05-23.
- Ferse, Sebastian C.A. (2010). "Poor Performance of Corals Transplanted onto Substrates of Short Durability". Restoration Ecology. 18 (4): 399–407. doi:10.1111/j.1526-100X.2010.00682.x.
Sources
- Allen, G.R; R. Steene (1994). Indo-Pacific Coral Reef Field Guide. ISBN 978-981-00-5687-2.
- Calfo, Anthony (2007). Book of Coral Propagation. ISBN 978-0-9802365-0-7.
- Colin, P.L.; C. Arneson (1995). Tropical Pacific Invertebrates. ISBN 978-0-9645625-0-9.
- Fagerstrom, J.A. (1987). The Evolution of Reef Communities. ISBN 978-0-471-81528-0.
- Gosliner, T.; D. Behrens; G. Williams (1996). Coral Reef Animals of the Indo-Pacific, Animals Life from Africa to Hawai'i (invertebrates). ISBN 978-0-930118-21-1.
- Nybakken, J.W. (2004). Marine Biology, An Ecological Approach. ISBN 978-0-8053-4582-7.
- Redhill, Surrey. Corals of the World: Biology and Field Guide.
- Segaloff, Nat; Paul Erickson (1991). A Reef Comes to Life. Creating an Undersea Exhibit. ISBN 978-0-531-10994-6.
- Sheppard, Charles R.C.; Davy, Simon K.; Pilling, Graham M. (25 June 2009). The Biology of Coral Reefs. OUP Oxford. ISBN 978-0-19-105734-2.
- Veron, J.E.N. (1993). Corals of Australia and the Indo-Pacific. ISBN 978-0-8248-1504-2.
- Wells, Susan. Coral Reefs of the World.
External links
| Wikispecies has information related to Coral |
| Wikimedia Commons has media related to |
- Coral Reefs The Ocean Portal by the Smithsonian Institution
- NOAA - Coral Reef Conservation Program
- NOAA CoRIS – Coral Reef Biology
- NOAA Office for Coastal Management - Fast Facts - Coral Reefs
- NOAA Ocean Service Education – Corals
- "What is a coral?". Stanford microdocs project. Retrieved 2017-02-04.

