GRK6
This gene encodes a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinase family, and is most highly similar to GRK4 and GRK5.[5][6][7] The protein phosphorylates the activated forms of G protein-coupled receptors to regulate their signaling.
Function
G protein-coupled receptor kinases phosphorylate activated G protein-coupled receptors, which promotes the binding of an arrestin protein to the receptor. Arrestin binding to phosphorylated, active receptor prevents receptor stimulation of heterotrimeric G protein transducer proteins, blocking their cellular signaling and resulting in receptor desensitization. Arrestin binding also directs receptors to specific cellular internalization pathways, removing the receptors from the cell surface and also preventing additional activation. Arrestin binding to phosphorylated, active receptor also enables receptor signaling through arrestin partner proteins. Thus the GRK/arrestin system serves as a complex signaling switch for G protein-coupled receptors.[8]
GRK6 and the closely related GRK5 phosphorylate receptors at sites that encourage arrestin-mediated signaling rather than arrestin-mediated receptor desensitization, internalization and trafficking (in contrast to GRK2 and GRK3, which have the opposite effect).[9][10] This difference is one basis for pharmacological biased agonism (also called functional selectivity), where a drug binding to a receptor may bias that receptor’s signaling toward a particular subset of the actions stimulated by that receptor.[11][12]
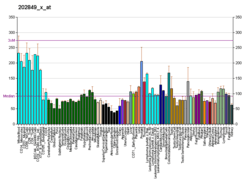
GRK6 is widely and relatively evenly expressed throughout the body, but with particularly high expression in immune cells.[6] GRK6 exists in three splice variants that differ in the carboxyl terminal region that regulates membrane association: one form is palmitoylated, another contains a lipid-binding polybasic domain, and the third is truncated and has neither.[13] In the mouse, GRK6 regulates the D2 dopamine receptor in the striatum region of the brain, and loss of GRK6 leads to increased sensitivity to psychostimulant drugs that act through dopamine.[14] Overexpression of GRK6 in the striatum in a rat model of Parkinson’s disease improves drug-induced movement disorder (tardive dyskinesia) symptoms arising from L-DOPA therapy.[15] In mouse immune cells, GRK6 is important for chemotaxis of B-lymphocytes and T-lymphocytes in response to the chemoattractant CXCL12,[16] and of neutrophils to sites of injury in response to leukotriene B4.[17]
References
- GRCh38: Ensembl release 89: ENSG00000198055 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000074886 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Benovic JL, Gomez J (1993). "Molecular cloning and expression of GRK6. A new member of the G protein-coupled receptor kinase family". J Biol Chem. 268 (26): 19521–19527. PMID 8366096.
- Haribabu B, Snyderman R (1993). "Identification of additional members of human G-protein-coupled receptor kinase multigene family". Proc Natl Acad Sci USA. 90 (20): 9398–9402. doi:10.1073/pnas.90.20.9398. PMC 47575. PMID 8415712.
- Premont RT, Inglese J, Lefkowitz RJ (1995). "Protein kinases that phosphorylate activated G protein-coupled receptors". FASEB J. 9 (2): 175–182. doi:10.1096/fasebj.9.2.7781920. PMID 7781920.
- Gurevich VV, Gurevich EV (2019). "GPCR Signaling Regulation: The Role of GRKs and Arrestins". Front Pharmacol. 10. doi:10.3389/fphar.2019.00125. PMC 6389790. PMID 30837883.
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ (2005). "Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling". Proc Natl Acad Sci USA. 102 (5): 1442–1447. doi:10.1073/pnas.0409532102. PMC 547874. PMID 15671181.
- Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ (2005). "Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor". Proc Natl Acad Sci USA. 102 (5): 1448–1453. doi:10.1073/pnas.0409534102. PMC 547876. PMID 15671180.
- Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ (2009). "Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands". Proc Natl Acad Sci USA. 106 (24): 9649–9654. doi:10.1073/pnas.0904361106. PMC 2689814. PMID 19497875.
- Choi M, Staus DP, Wingler LM, Ahn S, Pani B, Capel WD, Lefkowitz RJ (2018). "G protein-coupled receptor kinases (GRKs) orchestrate biased agonism at the β2-adrenergic receptor". Sci Signal. 11 (544): eaar7084. doi:10.1126/scisignal.aar7084. PMID 30131371.
- Premont RT, Macrae AD, Aparicio SA, Kendall HE, Welch JE, Lefkowitz RJ (1999). "The GRK4 subfamily of G protein-coupled receptor kinases. Alternative splicing, gene organization, and sequence conservation". J Biol Chem. 274 (41): 29381–29389. doi:10.1074/jbc.274.41.29381. PMID 10506199.
- Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, Premont RT (2003). "Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice". Neuron. 38 (2): 291–303. doi:10.1016/S0896-6273(03)00192-2. PMID 12718862.
- Ahmed MR, Berthet A, Bychkov E, Porras G, Li Q, Bioulac BH, Carl YT, Bloch B, Kook S, Aubert I, Dovero S, Doudnikoff E, Gurevich VV, Gurevich EV, Bezard E (2010). "Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson's disease". Sci Transl Med. 2 (28): 28ra28. doi:10.1126/scitranslmed.3000664. PMC 2933751. PMID 20410529.
- Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD (2002). "Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice". Proc Natl Acad Sci USA. 99 (11): 7478–7483. doi:10.1073/pnas.112198299. PMC 124256. PMID 12032308.
- Kavelaars A, Vroon A, Raatgever RP, Fong AM, Premont RT, Patel DD, Lefkowitz RJ, Heijnen CJ (2003). "Increased acute inflammation, leukotriene B4-induced chemotaxis, and signaling in mice deficient for G protein-coupled receptor kinase 6". J Immunol. 171 (11): 6128–6134. doi:10.4049/jimmunol.171.11.6128. PMID 14634128.
Further reading
- Bullrich F, Druck T, Kunapuli P, et al. (1995). "Chromosomal mapping of the genes GPRK5 and GPRK6 encoding G protein-coupled receptor kinases GRK5 and GRK6". Cytogenet. Cell Genet. 70 (3–4): 250–4. doi:10.1159/000134045. PMID 7789183.
- Stoffel RH, Randall RR, Premont RT, et al. (1994). "Palmitoylation of G protein-coupled receptor kinase, GRK6. Lipid modification diversity in the GRK family". J. Biol. Chem. 269 (45): 27791–4. PMID 7961702.
- Loudon RP, Benovic JL (1994). "Expression, purification, and characterization of the G protein-coupled receptor kinase GRK6". J. Biol. Chem. 269 (36): 22691–7. PMID 8077221.
- Benovic JL, Gomez J (1993). "Molecular cloning and expression of GRK6. A new member of the G protein-coupled receptor kinase family". J. Biol. Chem. 268 (26): 19521–7. PMID 8366096.
- Freedman NJ, Ament AS, Oppermann M, et al. (1997). "Phosphorylation and desensitization of human endothelin A and B receptors. Evidence for G protein-coupled receptor kinase specificity". J. Biol. Chem. 272 (28): 17734–43. doi:10.1074/jbc.272.28.17734. PMID 9211925.
- Loudon RP, Benovic JL (1997). "Altered activity of palmitoylation-deficient and isoprenylated forms of the G protein-coupled receptor kinase GRK6". J. Biol. Chem. 272 (43): 27422–7. doi:10.1074/jbc.272.43.27422. PMID 9341194.
- Stoffel RH, Inglese J, Macrae AD, et al. (1998). "Palmitoylation increases the kinase activity of the G protein-coupled receptor kinase, GRK6". Biochemistry. 37 (46): 16053–9. doi:10.1021/bi981432d. PMID 9819198.
- Premont RT, Claing A, Vitale N, et al. (1998). "β2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein". Proc. Natl. Acad. Sci. U.S.A. 95 (24): 14082–7. doi:10.1073/pnas.95.24.14082. PMC 24330. PMID 9826657.
- Milcent MD, Christophe T, Rabiet MJ, et al. (1999). "Overexpression of wild-type and catalytically inactive forms of GRK2 and GRK6 fails to alter the agonist-induced phosphorylation of the C5a receptor (CD88): evidence that GRK6 is autophosphorylated in COS-7 cells". Biochem. Biophys. Res. Commun. 259 (1): 224–9. doi:10.1006/bbrc.1999.0758. PMID 10334944.
- Lazari MF, Liu X, Nakamura K, et al. (1999). "Role of G protein-coupled receptor kinases on the agonist-induced phosphorylation and internalization of the follitropin receptor". Mol. Endocrinol. 13 (6): 866–78. doi:10.1210/me.13.6.866. PMID 10379886.
- Hall RA, Spurney RF, Premont RT, et al. (1999). "G protein-coupled receptor kinase 6A phosphorylates the Na(+)/H(+) exchanger regulatory factor via a PDZ domain-mediated interaction". J. Biol. Chem. 274 (34): 24328–34. doi:10.1074/jbc.274.34.24328. PMID 10446210.
- Brenninkmeijer CB, Price SA, López Bernal A, Phaneuf S (1999). "Expression of G-protein-coupled receptor kinases in pregnant term and non-pregnant human myometrium". J. Endocrinol. 162 (3): 401–8. doi:10.1677/joe.0.1620401. PMID 10467231.
- Pronin AN, Morris AJ, Surguchov A, Benovic JL (2000). "Synucleins are a novel class of substrates for G protein-coupled receptor kinases". J. Biol. Chem. 275 (34): 26515–22. doi:10.1074/jbc.M003542200. PMID 10852916.
- Tiruppathi C, Yan W, Sandoval R, et al. (2000). "G protein-coupled receptor kinase-5 regulates thrombin-activated signaling in endothelial cells". Proc. Natl. Acad. Sci. U.S.A. 97 (13): 7440–5. doi:10.1073/pnas.97.13.7440. PMC 16564. PMID 10861009.
- Zhou H, Yan F, Tai HH (2001). "Phosphorylation and desensitization of the human thromboxane receptor-alpha by G protein-coupled receptor kinases". J. Pharmacol. Exp. Ther. 298 (3): 1243–51. PMID 11504827.
- Blaukat A, Pizard A, Breit A, et al. (2001). "Determination of bradykinin B2 receptor in vivo phosphorylation sites and their role in receptor function". J. Biol. Chem. 276 (44): 40431–40. doi:10.1074/jbc.M107024200. PMID 11517230.
- Willets JM, Challiss RA, Nahorski SR (2002). "Endogenous G protein-coupled receptor kinase 6 Regulates M3 muscarinic acetylcholine receptor phosphorylation and desensitization in human SH-SY5Y neuroblastoma cells". J. Biol. Chem. 277 (18): 15523–9. doi:10.1074/jbc.M111217200. PMID 11856737.
- Grange-Midroit M, García-Sevilla JA, Ferrer-Alcón M, et al. (2002). "G protein-coupled receptor kinases, beta-arrestin-2 and associated regulatory proteins in the human brain: postmortem changes, effect of age and subcellular distribution". Brain Res. Mol. Brain Res. 101 (1–2): 39–51. doi:10.1016/S0169-328X(02)00144-4. PMID 12007830.