Tungsten disulfide
 | |
 Left: WS2 film on sapphire. Right: dark exfoliated WS2 film floating on water. | |
| Names | |
|---|---|
| IUPAC names
Tungsten disulfide Bis(sulfanylidene)tungsten | |
| Systematic IUPAC name
Dithioxotungsten | |
| Other names
Tungsten(IV) sulfide Tungstenite | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.032.027 |
| EC Number | 235-243-3 |
PubChem CID |
|
| |
| |
| Properties | |
| WS2 | |
| Molar mass | 247.98 g/mol |
| Appearance | blue-gray powder[1] |
| Density | 7.5 g/cm3, solid[1] |
| Melting point | 1,250 °C (2,280 °F; 1,520 K) decomposes[1] |
| slightly soluble | |
| Band gap | ~1 eV (indirect, bulk) ~1.8 eV (direct, monolayer)[2][3] |
| +5850·10−6 cm3/mol[4] | |
| Structure | |
| Molybdenite | |
| Trigonal prismatic (WIV) Pyramidal (S2−) | |
| Related compounds | |
Other anions |
Tungsten(IV) oxide, Tungsten diselenide |
Other cations |
Molybdenum disulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tungsten disulfide is the chemical compound with the formula WS2. It occurs naturally as the rare mineral tungstenite. This material is a component of certain catalysts used for hydrodesulfurization and hydrodenitrification.
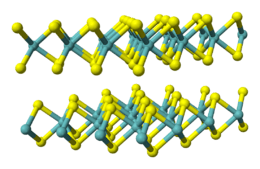
WS2 adopts a layered structure related to MoS2, with W atoms situated in trigonal prismatic coordination sphere. Owing to this layered structure, WS2 forms inorganic nanotubes, which were discovered on an example of WS2 in 1992.[5]
Properties
Bulk WS2 forms dark gray hexagonal crystals with a layered structure. Like the closely related MoS2, it exhibits properties of a dry lubricant. It is chemically fairly inert but is attacked by a mixture of nitric and hydrofluoric acids. When heated in oxygen-containing atmosphere, WS2 converts to tungsten trioxide. When heated in absence of oxygen, WS2 does not melt but decomposes to tungsten and sulfur, but only at 1250 °C.[1]
The material undergoes exfoliation by treatment with various reagents such as chlorosulfonic acid.[6]
Synthesis
WS2 is produced by a number of methods.[1][7] Many of these methods involve treating oxides with sources of sulfide or hydrosulfide, supplied as hydrogen sulfide or generated in situ. Other routes entail thermolysis of tungsten(VI) sulfides (e.g., (R4N)2WS4) or the equivalent (e.g., WS3).[7]
Freestanding WS2 films can be produced as follows. WS2 is deposited on a hydrophilic substrate, such as sapphire, and then coated with a polymer, such as polystyrene. After dipping the sample in water for a few minutes, the hydrophobic WS2 film spontaneously peels off.[8]
Applications
WS2 is used, in conjunction with other materials, as catalyst for hydrotreating of crude oil.[7]
Lamellar tungsten disulfide is used as a dry lubricant for fasteners, bearings, and molds under the brand name Dicronite.[9]
Research
Like MoS2, nanostructured WS2 is actively studied for potential applications, such as storage of hydrogen and lithium.[6] WS2 also catalyses hydrogenation of carbon dioxide:[6][10][11]
- CO2 + H2 → CO + H2O
Nanotubes
Tungsten disulfide is the first material which was found to form inorganic nanotubes, in 1992.[5] This ability is related to the layered structure of WS2, and macroscopic amounts of WS2 have been produced by the methods mentioned above.[7] WS2 nanotubes have been investigated as reinforcing agents to improve the mechanical properties of polymeric nanocomposites. In a study, WS2 nanotubes reinforced biodegradable polymeric nanocomposites of polypropylene fumarate (PPF) showed significant increases in the Young's modulus, compression yield strength, flexural modulus and flexural yield strength, compared to single- and multi-walled carbon nanotubes reinforced PPF nanocomposites, suggesting that WS2 nanotubes may be better reinforcing agents than carbon nanotubes.[12] The addition of WS2 nanotubes to epoxy resin improved adhesion, fracture toughness and strain energy release rate. The wear of the nanotubes-reinforced epoxy is lower than that of pure epoxy.[13] WS2 nanotubes were embedded into a poly(methyl methacrylate) (PMMA) nanofiber matrix via electrospinning. The nanotubes were well dispersed and aligned along fiber axis. The enhanced stiffness and toughness of PMMA fiber meshes by means of inorganic nanotubes addition may have potential uses as impact-absorbing materials, e.g. for ballistic vests.[14][15]
WS2 nanotubes are hollow and can be filled with another material, to preserve or guide it to a desired location, or to generate new properties in the filler material which is confined within a nanometer-scale diameter. To this goal, inorganic nanotube hybrids were made by filling WS2 nanotubes with molten lead, antimony or bithmuth iodide salt by a capillary wetting process, resulting in PbI2@WS2, SbI3@WS2 or BiI3@WS2 core–shell nanotubes.[16]
Nanosheets
WS2 can also exist in the form of atomically thin sheets.[17] Such materials exhibit room-temperature photoluminescence in the monolayer limit.[18]
References
| Wikimedia Commons has media related to Tungsten disulfide. |
- 1 2 3 4 5 Eagleson, Mary (1994). Concise encyclopedia chemistry. Walter de Gruyter. p. 1129. ISBN 978-3-11-011451-5.
- ↑ Yun, Won Seok; Han, S. W.; Hong, Soon Cheol; Kim, In Gee; Lee, J. D. (2012). "Thickness and strain effects on electronic structures of transition metal dichalcogenides: 2H-MX2 semiconductors (M = Mo, W; X = S, Se, Te)". Physical Review B. 85 (3): 033305. Bibcode:2012PhRvB..85c3305Y. doi:10.1103/PhysRevB.85.033305.
- 1 2 Sasaki, Shogo; Kobayashi, Yu; Liu, Zheng; Suenaga, Kazutomo; Maniwa, Yutaka; Miyauchi, Yuhei; Miyata, Yasumitsu (2016). "Growth and optical properties of Nb-doped WS2 monolayers". Applied Physics Express. 9 (7): 071201. Bibcode:2016APExp...9g1201S. doi:10.7567/APEX.9.071201.

- ↑ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.136. ISBN 1439855110.
- 1 2 Tenne R, Margulis L, Genut M, Hodes G (1992). "Polyhedral and cylindrical structures of tungsten disulphide". Nature. 360 (6403): 444–446. Bibcode:1992Natur.360..444T. doi:10.1038/360444a0.
- 1 2 3 Bhandavat, R.; David, L.; Singh, G. (2012). "Synthesis of Surface-Functionalized WS2 Nanosheets and Performance as Li-Ion Battery Anodes". The Journal of Physical Chemistry Letters. 3 (11): 1523. doi:10.1021/jz300480w. PMID 26285632.
- 1 2 3 4 Panigrahi, Pravas Kumar; Pathak, Amita (2008). "Microwave-assisted synthesis of WS2 nanowires through tetrathiotungstate precursors" (free download). Sci. Technol. Adv. Mater. 9 (4): 045008. Bibcode:2008STAdM...9d5008P. doi:10.1088/1468-6996/9/4/045008. PMC 5099650. PMID 27878036.
- ↑ Yu, Yang; Fong, Patrick W. K.; Wang, Shifeng; Surya, Charles (2016). "Fabrication of WS2/GaN p-n Junction by Wafer-Scale WS2 Thin Film Transfer". Scientific Reports. 6: 37833. Bibcode:2016NatSR...637833Y. doi:10.1038/srep37833. PMC 5126671. PMID 27897210.
- ↑ French, Lester Gray, ed. (1967). "Dicronite". Machinery. Vol. 73. Machinery Publications Corporation. p. 101.
- ↑ Lassner, Erik; Schubert, Wolf-Dieter (1999). Tungsten: properties, chemistry, technology of the element, alloys, and chemical compounds. Springer. pp. 374–. ISBN 978-0-306-45053-2.
- ↑ Engineer making rechargeable batteries with layered nanomaterials. Science Daily (2013-01-016)
- ↑ Lalwani, Gaurav (September 2013). "Tungsten disulfide nanotubes reinforced biodegradable polymers for bone tissue engineering". Acta Biomaterialia. 9 (9): 8365–8373. doi:10.1016/j.actbio.2013.05.018. PMC 3732565. PMID 23727293.
- ↑ Zohar, E.; et al. (2011). "The Mechanical and Tribological Properties of Epoxy Nanocomposites with WS2 Nanotubes". Sensors & Transducers Journal. 12 (Special Issue): 53–65.
- ↑ Reddy, C. S.; Zak, A. & Zussman, E. (2011). "WS2 nanotubes embedded in PMMA nanofibers as energy absorptive material". J. Mater. Chem. 21 (40): 16086–16093. doi:10.1039/C1JM12700D.
- ↑ Nano-Armor: Protecting the Soldiers of Tomorrow. Physorg.com (2005-12-10). Retrieved on 2016-01-20.
- ↑ Kreizman, Ronen; Enyashin, Andrey N.; Deepak, Francis Leonard; Albu-Yaron, Ana; Popovitz-Biro, Ronit; Seifert, Gotthard; Tenne, Reshef (2010). "Synthesis of Core-Shell Inorganic Nanotubes". Adv. Funct. Mater. 20 (15): 2459–2468. doi:10.1002/adfm.201000490.
- ↑ Coleman, J. N.; Lotya, M.; O'Neill, A.; Bergin, S. D.; King, P. J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R. J.; Shvets, I. V.; Arora, S. K.; Stanton, G.; Kim, H.-Y.; Lee, K.; Kim, G. T.; Duesberg, G. S.; Hallam, T.; Boland, J. J.; Wang, J. J.; Donegan, J. F.; Grunlan, J. C.; Moriarty, G.; Shmeliov, A.; Nicholls, R. J.; Perkins, J. M.; Grieveson, E. M.; Theuwissen, K.; McComb, D. W.; et al. (2011). "Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials". Science. 331 (6017): 568. Bibcode:2011Sci...331..568C. doi:10.1126/science.1194975. PMID 21292974.
- ↑ Gutiérrez, Humberto R.; Perea-López, Nestor; Elías, Ana Laura; Berkdemir, Ayse; Wang, Bei; Lv, Ruitao; López-Urías, Florentino; Crespi, Vincent H.; Terrones, Humberto; Terrones, Mauricio (2013). "Extraordinary Room-Temperature Photoluminescence in Triangular WS2 Monolayers". Nano Letters. 13 (8): 3447. arXiv:1208.1325. Bibcode:2013NanoL..13.3447G. doi:10.1021/nl3026357. PMID 23194096.