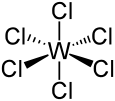
Tungsten hexachloride
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Tungsten hexachloride Tungsten(VI) chloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ECHA InfoCard | 100.032.980 | ||
| EC Number | 236-293-9 | ||
PubChem CID |
|||
| RTECS number | YO7710000 | ||
| |||
| |||
| Properties | |||
| WCl6 | |||
| Molar mass | 396.61 g/mol | ||
| Appearance | dark blue crystals, moisture sensitive | ||
| Density | 3.52 g/cm3 | ||
| Melting point | 275 °C (527 °F; 548 K) | ||
| Boiling point | 346.7 °C (656.1 °F; 619.8 K) | ||
| hydrolyzes | |||
| Solubility in chlorocarbons | soluble | ||
| −71.0·10−6 cm3/mol | |||
| Structure | |||
| α:rhombohedral, β: hexagonal | |||
| octahedral | |||
| 0 D | |||
| Hazards | |||
| Main hazards | oxidizer; hydrolysis releases HCl | ||
| Related compounds | |||
Other anions |
Tungsten hexafluoride Tungsten hexabromide | ||
Other cations |
Molybdenum(V) chloride Chromyl chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds.[1] Other examples of charge-neutral hexachlorides are ReCl6 and MoCl6. The highly volatile WF6 is also known.
As a d0 ion, W(VI) forms diamagnetic derivatives. The hexachloride is octahedral with equivalent W–Cl distances of 2.24–2.26 Å.[2]
Preparation
Tungsten hexachloride can be prepared by chlorinating tungsten metal in a sealed tube at 600 °C: [3]
- W + 3 Cl2 → WCl6
Properties and Reactions
Tungsten (VI) chloride is a blue-black solid at room temperature. At lower temperatures, it becomes wine-red in color. A red form of the compound can be made by rapidly condensing its vapor, which reverts to the blue-black form on gentle heating. It is readily hydrolyzed, even by moist air, giving the orange oxychlorides WOCl4 and WO2Cl2, and subsequently, tungsten trioxide. WCl6 is soluble in carbon disulfide, carbon tetrachloride, and phosphorus oxychloride.[3]
Methylation with trimethylaluminium affords hexamethyl tungsten:
- WCl6 +3 Al2(CH3)6 → W(CH3)6 + 3 Al2(CH3)4Cl2
Treatment with butyl lithium affords a reagent that is useful for deoxygenation of epoxides.[4]
The chloride ligands in WCl6 can be replaced by many anionic ligands including: Br−, NCS−, and RO− (R = alkyl, aryl).
Reduction of WCl6 gives, sequentially, tungsten(V) chloride and tungsten(IV) chloride.
Safety considerations
WCl6 is an aggressively corrosive oxidant, and hydrolyzes to release hydrogen chloride.
References
- ↑ J. W. Herndon (2004). "Tungsten(VI) Chloride". Encyclopedia of Reagents for Organic Synthesis. Wiley. doi:10.1002/047084289. .
- ↑ J. C. Taylor, P. W. Wilson (1974). "The Structure of β-Tungsten Hexachloride by Powder Neutron and X-ray Diffraction". Acta Crystallographica. B30: 1216–1220. doi:10.1107/S0567740874004572. .
- 1 2 M. H. Lietzke; M. L. Holt (1950). "Tungsten(VI) Chloride (Tungsten Hexachloride)". Inorganic Syntheses. 3: 163. doi:10.1002/9780470132340.ch4.
- ↑ M. A. Umbreit, K. B. Sharpless (1990). "Deoxygenation of Epoxides with Lower Valent Tungsten Halides: trans-Cyclododecene". Organic Syntheses. ; Collective Volume, 7, p. 121