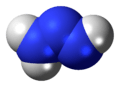
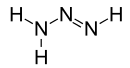Triazene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Triazene | |||
| Systematic IUPAC name
Triaz-1-ene[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| 49028 | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| H3N3 | |||
| Molar mass | 45.05 g·mol−1 | ||
| Hazards | |||
| NFPA 704 | |||
| Related compounds | |||
Other anions |
Triphosphane | ||
Related Binary azanes |
ammonia diazane triazane | ||
Related compounds |
Diazene Tetrazene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Triazene, also known as triazanylene, is an unsaturated inorganic compound having the chemical formula N3H3. It has one double bond, and is the second-simplest member of the azene class of hydronitrogen compounds, and is not found in nature. It is also the name given to the functional group consisting of an amine directly bonding to an azo group, i.e. with the linkage R1R2N-N=NR3 where R1, R2 and R3 are substituents. The functional group is also called a diazoamino group (but only one of the two substituents R1 and R3 may be hydrogen) because it is related to a diazo group.
Properties
At room temperature, triazene is a gas and, as with many other azenes, it is also coloured with a strong and unpleasant smell. Triazene has a higher density and boiling point than diazene due to its greater mass. It has a slightly lower boiling point than triazane and is thus more volatile. It has strong polar bonds, and the molecule has a large dipole moment due to its reduced symmetry. Triazene has the same empirical formula as cyclotriazane but their atoms are connected in different ways, making these molecules structural isomers. Triazene is an electron-rich functional group and is capable to adsorb metallic[2] and organic[3] cations.
Medical uses
Some anti-cancer medications are called triazenes because they contain a triazene functional group. The triazenes are a group of alkylating agents used to battle cancer cells.[4] Examples include dacarbazine and temozolomide. They work by methylating guanine at the O-6 and N-7 position.
Production and Derivatives
To date, the only proven method to produce unsubstituted triazene is the spontaneous decomposition of tetrazane into triazene and ammonia.
However substituted analogues are prepared from the N-coupling reaction between diazonium salts and primary or secondary amines.
For instance, a well-known example of triazene is a diphenyl derivative,[5] PhNH-N=NPh (m.p. 100 °C, CAS #136-35-6).
Diphenyl triazene is prepared from the reaction of phenyldiazonium salt and a primary amine (aniline) in the presence of a mineral base (sodium carbonate) or buffering reagent (sodium acetate) :[6]
- PhN2+ + PhNH2 → PhNHN=NPh + H+
This bis-triazene analogue of Torger's base is obtained from N-coupling reaction between a bis-diazonium salt and a secondary amine (N-Methylaniline).[7]
Polymeric triazenes are also produced and applied as conductive and absorbent materials.[3]
Reactions
Triazenes have been used as an in situ source of diazonium.[6] Triazenes decompose in the presence of protonating or alkylating agents into quaternary amines and diazonium salts.[6] A strategy for the protection and deprotection of sensitive secondary amines is based on this principle.[8]
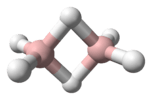
Triazenes can be reacted with sodium sulfide in the presence of trichloroacetic acid to give the corresponding thiophenols.[9][7]
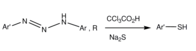 Triazene to thiophenol conversion
Triazene to thiophenol conversion
Triazene group can be converted into functionalized lactams,[10] triazoles, dibenzopyranones, and coumarins, or be utilized as a substrate for perfluoroalkylation[7] and diazenylation reactions.[11][12] In another example, the synthesis of cinnoline was accomplished by Richter reaction of triazene-masked diazonium ion.
References
- ↑ "triazene (CHEBI:35468)". Chemical Entities of Biological Interest. EMBL-EBI.
- ↑ Rofouei, Mohammad k; Soleymani, Reza; Aghaei, Abolfazl; Mirzaei, Mahmoud. "Synthesis, vibrational, electrostatic potential and NMR studies of (E and Z) 1-(4-chloro-3-nitrophenyl)-3-(2-methoxyphenyl)triazene: Combined experimental and DFT approaches". Journal of Molecular Structure. 1125: 247–259. Bibcode:2016JMoSt1125..247R. doi:10.1016/j.molstruc.2016.06.053.
- 1 2 Khazaei, Ardeshir; Kazem-Rostami, Masoud; Zare, Abdolkarim; Moosavi-Zare, Ahmad Reza; Sadeghpour, Mahdieh; Afkhami, Abbas (2013-09-15). "Synthesis, characterization, and application of a triazene-based polysulfone as a dye adsorbent". Journal of Applied Polymer Science. 129 (6): 3439–3446. doi:10.1002/app.39069. ISSN 1097-4628.
- ↑ Vajs, Jure; Steiner, Ivana; Brozovic, Anamaria; Pevec, Andrej; Ambriović-Ristov, Andreja; Matković, Marija; Piantanida, Ivo; Urankar, Damijana; Osmak, Maja. "The 1,3-diaryltriazenido(p-cymene)ruthenium(II) complexes with a high in vitro anticancer activity". Journal of Inorganic Biochemistry. 153: 42–48. doi:10.1016/j.jinorgbio.2015.09.005.
- ↑ Hartman, W. W.; Dickey, J. B. (1934). "Diazoaminobenzene". Organic Syntheses. 14: 24. ; Collective Volume, 2, p. 163
- 1 2 3 Kazem-Rostami, Masoud; Khazaei, Ardeshir; Moosavi-Zare, Ahmad; Bayat, Mohammad; Saednia, Shahnaz (August 2012). "Novel One-Pot Synthesis of Thiophenols from Related Triazenes under Mild Conditions". Synlett. 23 (13): 1893–1896. doi:10.1055/s-0032-1316557. ISSN 0936-5214.
- 1 2 3 Kazem-Rostami, Masoud (August 2017). "Facile Preparation of Λ-Shaped Building Blocks: Hünlich Base Derivatization". Synlett. 28 (13): 1641–1645. doi:10.1055/s-0036-1588180. ISSN 0936-5214.
- ↑ Lazny, R.; Poplawski, J.; Köbberling, J.; Enders, D.; Bräse, S. (1999). "Triazenes: A Useful Protecting Strategy for Sensitive Secondary Amines". Synlett. 1999 (8): 1304–6. doi:10.1055/s-1999-2803.
- ↑ Kazem-Rostami, M.; Khazaei, A.; Moosavi-Zare, A. R.; Bayat, M.; Saednia, S. (2012). "Novel One-Pot Synthesis of Thiophenols from Related Triazenes under Mild Conditions". Synlett. 23 (13): 1893–6. doi:10.1055/s-0032-1316557.
- ↑ Petrović, Martina; Scarpi, Dina; Nieger, Martin; Jung, Nicole; Occhiato, Ernesto G.; Bräse, Stefan (2017-01-30). "Oxidation of diazenyl-protected N-heterocycles – a new entry to functionalized lactams". RSC Adv. 7 (16): 9461–9464. doi:10.1039/c6ra26546d. ISSN 2046-2069.
- ↑ Zhang, Yonghong; Hu, Haiyan; Liu, Chen-Jiang; Cao, Dawei; Wang, Bin; Sun, Yadong; Abdukader, Ablimit (2017-01-01). "Highly Efficient Brønsted Acidic Ionic Liquid Promoted Direct Diazenylation of Pyrazolones with Aryltriazenes under Mild Conditions". Asian Journal of Organic Chemistry. 6 (1): 102–107. doi:10.1002/ajoc.201600475. ISSN 2193-5815.
- ↑ Cao, Dawei; Zhang, Yonghong; Liu, Chenjiang; Wang, Bin; Sun, Yadong; Abdukadera, Ablimit; Hu, Haiyan; Liu, Qiang (2016-05-06). "Ionic Liquid Promoted Diazenylation of N-Heterocyclic Compounds with Aryltriazenes under Mild Conditions". Organic Letters. 18 (9): 2000–2003. doi:10.1021/acs.orglett.6b00605. ISSN 1523-7060.