Symbiogenesis
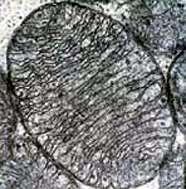
Symbiogenesis, or endosymbiotic theory, is an evolutionary theory of the origin of eukaryotic cells from prokaryotic organisms, first articulated in 1905 and 1910 by the Russian botanist Konstantin Mereschkowski, and advanced and substantiated with microbiological evidence by Lynn Margulis in 1967. It holds that the organelles distinguishing eukaryote cells evolved through symbiosis of individual single-celled prokaryotic (bacteria and archaea).
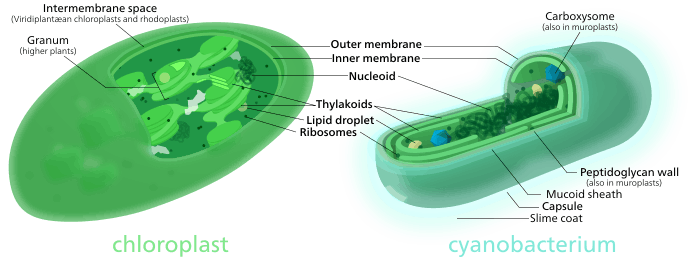
The theory holds that mitochondria, plastids such as chloroplasts, and possibly other organelles of eukaryotic cells represent formerly free-living prokaryotes taken one inside the other in endosymbiosis. In more detail, mitochondria appear to be related to Rickettsiales proteobacteria, and chloroplasts to nitrogen-fixing filamentous cyanobacteria.
Among the many lines of evidence supporting symbiogenesis are that new mitochondria and plastids are formed only through binary fission, and that cells cannot create new ones otherwise; that the transport proteins called porins are found in the outer membranes of mitochondria, chloroplasts and bacterial cell membranes; that cardiolipin is found only in the inner mitochondrial membrane and bacterial cell membranes; and that some mitochondria and plastids contain single circular DNA molecules similar to the chromosomes of bacteria.
History
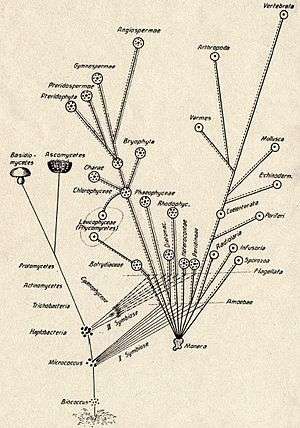
The theory of symbiogenesis (from Greek: σύν syn "together", βίος bios "life", and γένεσις genesis "origin, birth") was first outlined by the Russian botanist Konstantin Mereschkowski in his 1905 work, The nature and origins of chromatophores in the plant kingdom, and then elaborated in his 1910 The Theory of Two Plasms as the Basis of Symbiogenesis, a New Study of the Origins of Organisms.[2][3][4] Mereschkowski was familiar with work by botanist Andreas Schimper, who had observed in 1883 that the division of chloroplasts in green plants closely resembled that of free-living cyanobacteria, and who had himself tentatively proposed (in a footnote) that green plants had arisen from a symbiotic union of two organisms.[5] In 1918 the French scientist Paul Portier published Les Symbiotes in which he claimed that the mitochondria originated from a symbiosis process.[6][7] Ivan Wallin advocated the idea of an endosymbiotic origin of mitochondria in the 1920s.[8][9]
The Russian botanist Boris Kozo-Polyansky was the first to explain the theory in terms of Darwinian evolution.[10] In his 1924 book Novyi printzip biologii. Ocherk teorii simbiogeneza (The new principle of biology. Essay on the theory of symbiogenesis; translated to English as Symbiogenesis: A New Principle of Evolution in 2010[11]), he wrote, "The theory of symbiogenesis is a theory of selection relying on the phenomenon of symbiosis."[12] These theories were initially dismissed or ignored. More detailed electron microscopic comparisons between cyanobacteria and chloroplasts (for example studies by Hans Ris published in 1961[13]), combined with the discovery that plastids and mitochondria contain their own DNA[14] (which by that stage was recognized to be the hereditary material of organisms) led to a resurrection of the idea in the 1960s.
The theory was advanced and substantiated with microbiological evidence by Lynn Margulis in a 1967 paper, On the origin of mitosing cells.[15] In her 1981 work Symbiosis in Cell Evolution she argued that eukaryotic cells originated as communities of interacting entities, including endosymbiotic spirochaetes that developed into eukaryotic flagella and cilia. This last idea has not received much acceptance, because flagella lack DNA and do not show ultrastructural similarities to bacteria or archaea (see also: Evolution of flagella and Prokaryotic cytoskeleton). According to Margulis and Dorion Sagan,[16] "Life did not take over the globe by combat, but by networking" (i.e., by cooperation). The possibility that peroxisomes may have an endosymbiotic origin has also been considered, although they lack DNA. Christian de Duve proposed that they may have been the first endosymbionts, allowing cells to withstand growing amounts of free molecular oxygen in the Earth's atmosphere. However, it now appears that peroxisomes may be formed de novo, contradicting the idea that they have a symbiotic origin.[17]

From endosymbionts to organelles
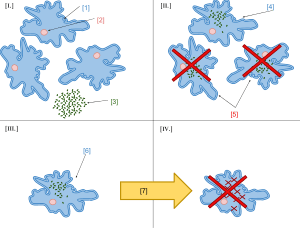
According to Keeling and Archibald,[18] the usual way to distinguish organelles from endosymbionts is by their reduced genome sizes. As an endosymbiont evolves into an organelle, most of their genes are transferred to the host cell genome.[19] The host cell and organelle need to develop a transport mechanism that enables the return of the protein products needed by the organelle but now manufactured by the cell. Cyanobacteria and α-proteobacteria are the most closely related free-living organisms to plastids and mitochondria respectively.[20] Both cyanobacteria and α-proteobacteria maintain a large (>6Mb) genome encoding thousands of proteins.[20] Plastids and mitochondria exhibit a dramatic reduction in genome size when compared to their bacterial relatives.[20] Chloroplast genomes in photosynthetic organisms are normally 120-200kb[21] encoding 20-200 proteins[20] and mitochondrial genomes in humans are approximately 16kb and encode 37 genes, 13 of which are proteins.[22] Using the example of the freshwater amoeboid, however, Paulinella chromatophora, which contains chromatophores found to be evolved from cyanobacteria, Keeling and Archibald argue that this is not the only possible criterion; another is that the host cell has assumed control of the regulation of the former endosymbiont's division, thereby synchronizing it with the cell's own division.[18] Nowack and her colleagues[23] performed gene sequencing on the chromatophore (1.02 Mb) and found that only 867 proteins were encoded by these photosynthetic cells. Comparisons with their closest free living cyanobacteria of the genus Synechococcus (having a genome size 3 Mb, with 3300 genes) revealed that chromatophores underwent a drastic genome shrinkage. Chromatophores contained genes that were accountable for photosynthesis but were deficient in genes that could carry out other biosynthetic functions; this observation suggests that these endosymbiotic cells are highly dependent on their hosts for their survival and growth mechanisms. Thus, these chromatophores were found to be non-functional for organelle-specific purposes when compared to mitochondria and plastids. This distinction could have promoted the early evolution of photosynthetic organelles.
The loss of genetic autonomy, that is, the loss of many genes from endosymbionts, occurred very early in evolutionary time.[24] Taking into account the entire original endosymbiont genome, there are three main possible fates for genes over evolutionary time. The first fate involves the loss of functionally redundant genes,[24] in which genes that are already represented in the nucleus are eventually lost. The second fate involves the transfer of genes to the nucleus.[20][24][25][26][27] The loss of autonomy and integration of the endosymbiont with its host can be primarily attributed to nuclear gene transfer.[27] As organelle genomes have been greatly reduced over evolutionary time, nuclear genes have expanded and become more complex.[20] As a result, many plastid and mitochondrial processes are driven by nuclear encoded gene products.[20] In addition, many nuclear genes originating from endosymbionts have acquired novel functions unrelated to their organelles.[20][27]
The mechanisms of gene transfer are not fully known; however, multiple hypotheses exist to explain this phenomenon. The cDNA hypothesis involves the use of messenger RNA (mRNAs) to transport genes from organelles to the nucleus where they are converted to cDNA and incorporated into the genome.[20][25] The cDNA hypothesis is based on studies of the genomes of flowering plants. Protein coding RNAs in mitochondria are spliced and edited using organelle-specific splice and editing sites. Nuclear copies of some mitochondrial genes, however, do not contain organelle-specific splice sites, suggesting a processed mRNA intermediate. The cDNA hypothesis has since been revised as edited mitochondrial cDNAs are unlikely to recombine with the nuclear genome and are more likely to recombine with their native mitochondrial genome. If the edited mitochondrial sequence recombines with the mitochondrial genome, mitochondrial splice sites would no longer exist in the mitochondrial genome. Any subsequent nuclear gene transfer would therefore also lack mitochondrial splice sites.[20]
The bulk flow hypothesis is the alternative to the cDNA hypothesis, stating that escaped DNA, rather than mRNA, is the mechanism of gene transfer.[20][25] According to this hypothesis, disturbances to organelles, including autophagy (normal cell destruction), gametogenesis (the formation of gametes), and cell stress, release DNA which is imported into the nucleus and incorporated into the nuclear DNA using non-homologous end joining (repair of double stranded breaks).[25] For example, in the initial stages of endosymbiosis, due to a lack of major gene transfer, the host cell had little to no control over the endosymbiont. The endosymbiont underwent cell division independently of the host cell, resulting in many "copies" of the endosymbiont within the host cell. Some of the endosymbionts lysed (burst), and high levels of DNA were incorporated into the nucleus. A similar mechanism is thought to occur in tobacco plants, which show a high rate of gene transfer and whose cells contain multiple chloroplasts.[24] In addition, the bulk flow hypothesis is also supported by the presence of non-random clusters of organelle genes, suggesting the simultaneous movement of multiple genes.[25]
In 2015, the biologist Roberto Cazzolla Gatti provided evidence for a variant theory,[28] endogenosymbiosis, in which not only are organelles endosymbiotic, but that pieces of genetic material from symbiotic parasites ("gene carriers" such as viruses, retroviruses and bacteriophages), are included in the host's nuclear DNA, changing the host's gene expression and contributing to the process of speciation.[29]
Molecular and biochemical evidence suggests that mitochondria are related to Rickettsiales proteobacteria (in particular, the SAR11 clade,[30][31] or close relatives), and that chloroplasts are related to nitrogen-fixing filamentous cyanobacteria.[32][33]
Organellar genomes
Plastomes and mitogenomes
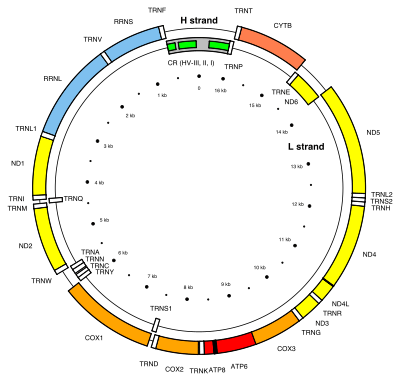
The third and final possible fate of endosymbiont genes is that they remain in the organelles. Plastids and mitochondria, although they have lost much of their genomes, retain genes encoding rRNAs, tRNAs, proteins involved in redox reactions, and proteins required for transcription, translation, and replication.[20][21][24] There are many hypotheses to explain why organelles retain a small portion of their genome; however no one hypothesis will apply to all organisms[24] and the topic is still quite controversial.[20] The hydrophobicity hypothesis states that highly hydrophobic (water hating) proteins (such as the membrane bound proteins involved in redox reactions) are not easily transported through the cytosol and therefore these proteins must be encoded in their respective organelles.[20][24] The code disparity hypothesis states that the limit on transfer is due to differing genetic codes and RNA editing between the organelle and the nucleus.[24] The redox control hypothesis states that genes encoding redox reaction proteins are retained in order to effectively couple the need for repair and the synthesis of these proteins.[20][21][24] For example, if one of the photosystems is lost from the plastid, the intermediate electron carriers may lose or gain too many electrons, signalling the need for repair of a photosystem.[21] The time delay involved in signalling the nucleus and transporting a cytosolic protein to the organelle results in the production of damaging reactive oxygen species.[20][21][24] The final hypothesis states that the assembly of membrane proteins, particularly those involved in redox reactions, requires coordinated synthesis and assembly of subunits; however, translation and protein transport coordination is more difficult to control in the cytoplasm.[24]
Non-photosynthetic plastid genomes
The majority of the genes in the mitochondria and plastids are related to the expression (transcription, translation and replication) of genes encoding proteins involved in either photosynthesis (in plastids) or cellular respiration (in mitochondria).[20][21][24] One might predict that the loss of photosynthesis or cellular respiration would allow for the complete loss of the plastid genome or the mitochondrial genome respectively.[24] While there are numerous examples of mitochondrial descendants (mitosomes and hydrogenosomes) that have lost their entire organellar genome,[34] non-photosynthetic plastids tend to retain a small genome.[24] There are two main hypotheses to explain this occurrence:
The essential tRNA hypothesis notes that there have been no documented functional plastid-to-nucleus gene transfers of genes encoding RNA products (tRNAs and rRNAs). As a result, plastids must make their own functional RNAs or import nuclear counterparts. The genes encoding tRNA-Glu and tRNA-fmet, however, appear to be indispensable. The plastid is responsible for haem biosynthesis, which requires plastid encoded tRNA-Glu (from the gene trnE) as a precursor molecule. Like other genes encoding RNAs, trnE cannot be transferred to the nucleus. In addition, it is unlikely trnE could be replaced by a cytosolic tRNA-Glu as trnE is highly conserved; single base changes in trnE have resulted in the loss of haem synthesis. The gene for tRNA-formylmethionine (tRNA-fmet) is also encoded in the plastid genome and is required for translation initiation in both plastids and mitochondria. A plastid is required to continue expressing the gene for tRNA-fmet so long as the mitochondrion is translating proteins.[24]
The limited window hypothesis offers a more general explanation for the retention of genes in non-photosynthetic plastids.[35] According to the bulk flow hypothesis, genes are transferred to the nucleus following the disturbance of organelles.[25] Disturbance was common in the early stages of endosymbiosis, however, once the host cell gained control of organelle division, eukaryotes could evolve to have only one plastid per cell. Having only one plastid severely limits gene transfer[24] as the lysis of the single plastid would likely result in cell death.[24][35] Consistent with this hypothesis, organisms with multiple plastids show an 80-fold increase in plastid-to-nucleus gene transfer compared to organisms with single plastids.[35]
Evidence
There are many lines of evidence that mitochondria and plastids including chloroplasts arose from bacteria.[36][37][38][39][40]
- New mitochondria and plastids are formed only through binary fission, the form of cell division used by bacteria and archaea.[41]
- If a cell's mitochondria or chloroplasts are removed, the cell does not have the means to create new ones.[42] For example, in some algae, such as Euglena, the plastids can be destroyed by certain chemicals or prolonged absence of light without otherwise affecting the cell. In such a case, the plastids will not regenerate.
- Transport proteins called porins are found in the outer membranes of mitochondria and chloroplasts and are also found in bacterial cell membranes.[43][44][45]
- A membrane lipid cardiolipin is exclusively found in the inner mitochondrial membrane and bacterial cell membranes.[46]
- Some mitochondria and some plastids contain single circular DNA molecules that are similar to the DNA of bacteria both in size and structure.[47]
- Genome comparisons suggest a close relationship between mitochondria and Rickettsial bacteria.[48]
- Genome comparisons suggest a close relationship between plastids and cyanobacteria.[49]
- Many genes in the genomes of mitochondria and chloroplasts have been lost or transferred to the nucleus of the host cell. Consequently, the chromosomes of many eukaryotes contain genes that originated from the genomes of mitochondria and plastids.[47]
- Mitochondrial and plastid ribosomes are more similar to those of bacteria (70S) than those of eukaryotes.[50]
- Proteins created by mitochondria and chloroplasts use N-formylmethionine as the initiating amino acid, as do proteins created by bacteria but not proteins created by eukaryotic nuclear genes or archaea.[51][52]
Secondary endosymbiosis
Primary endosymbiosis involves the engulfment of a cell by another free living organism. Secondary endosymbiosis occurs when the product of primary endosymbiosis is itself engulfed and retained by another free living eukaryote. Secondary endosymbiosis has occurred several times and has given rise to extremely diverse groups of algae and other eukaryotes. Some organisms can take opportunistic advantage of a similar process, where they engulf an alga and use the products of its photosynthesis, but once the prey item dies (or is lost) the host returns to a free living state. Obligate secondary endosymbionts become dependent on their organelles and are unable to survive in their absence (for a review see McFadden 2001[53]). RedToL, the Red Algal Tree of Life Initiative funded by the National Science Foundation highlights the role red algae or Rhodophyta played in the evolution of our planet through secondary endosymbiosis.
One possible secondary endosymbiosis in process has been observed by Okamoto & Inouye (2005). The heterotrophic protist Hatena behaves like a predator until it ingests a green alga, which loses its flagella and cytoskeleton, while Hatena, now a host, switches to photosynthetic nutrition, gains the ability to move towards light and loses its feeding apparatus.[54]
The process of secondary endosymbiosis left its evolutionary signature within the unique topography of plastid membranes. Secondary plastids are surrounded by three (in euglenophytes and some dinoflagellates) or four membranes (in haptophytes, heterokonts, cryptophytes, and chlorarachniophytes). The two additional membranes are thought to correspond to the plasma membrane of the engulfed alga and the phagosomal membrane of the host cell. The endosymbiotic acquisition of a eukaryote cell is represented in the cryptophytes; where the remnant nucleus of the red algal symbiont (the nucleomorph) is present between the two inner and two outer plastid membranes.
Despite the diversity of organisms containing plastids, the morphology, biochemistry, genomic organisation, and molecular phylogeny of plastid RNAs and proteins suggest a single origin of all extant plastids – although this theory is still debated.[55][56]
Some species including Pediculus humanus (lice) have multiple chromosomes in the mitochondrion. This and the phylogenetics of the genes encoded within the mitochondrion suggest that mitochondria have multiple ancestors, that these were acquired by endosymbiosis on several occasions rather than just once, and that there have been extensive mergers and rearrangements of genes on the several original mitochondrial chromosomes.[57]
Date
The question of when the transition from prokaryotic to eukaryotic form occurred and when the first crown-group eukaryotes appeared on earth is still unresolved. The oldest known body fossils that can be positively assigned to the Eukaryota are acanthomorphic acritarchs from the 1631±1 Ma Deonar Formation of India (lower Vindhyan Supergroup) of India.[58] These fossils can still be identified as derived post-nuclear eukaryotes with a sophisticated, morphology-generating cytoskeleton sustained by mitochondria.[59] This fossil evidence indicates that endosymbiotic acquisition of alphaproteobacteria must have occurred before 1.6 Ga. Molecular clocks have also been used to estimate the last eukaryotic common ancestor (LECA, however these methods have large inherent uncertainty and give a wide range of dates. Reasonable results for LECA include the estimate of c. 1800 Mya.[60] A 2300 Mya estimate[61] also seems reasonable and has the added attraction of coinciding with one of the most pronounced biogeochemical perturbations in Earth history (the Great Oxygenation Event). The marked increase in atmospheric oxygen concentrations during the early Palaeoproterozoic Great Oxidation Event has been invoked as a contributing cause of eukaryogenesis – by inducing the evolution of oxygen-detoxifying mitochondria.[62] Alternatively, the Great Oxidation Event might be a consequence of eukaryogenesis and its impact on the export and burial of organic carbon.[63]
See also
- Angomonas deanei, a protozoan that harbours an obligate bacterial symbiont
- Hatena arenicola, a species that appears to be in the process of acquiring an endosymbiont
- Hydrogen hypothesis
- James A. Lake
- Kleptoplasty
- Mixotricha paradoxa, which itself is a symbiont, contains endosymbiotic bacteria
- Numt, abbreviation of "nuclear mitochondrial DNA"
- Parasite Eve, fiction about endosymbiosis
- Protocell
- Viral eukaryogenesis, hypothesis that the cell nucleus originated from endosymbiosis
References
- ↑ "Mereschkowsky's Tree of Life". Scientific American. Retrieved 1 May 2017.
- ↑ Mereschkowski, K. (15 September 1905). "Über Natur und Ursprung der Chromatophoren im Pflanzenreiche" [On the nature and origin of chromatophores in the plant kingdom]. Biologisches Centralblatt (in German). 25 (18): 593–604.
- ↑ See:
- Mereschkowski, Konstantin (15 April 1910). "Theorie der zwei Plasmaarten als Grundlage der Symbiogenesis, einer neuen Lehre von der Entstehung der Organismen" [Theory of two types of plasma as the basis of symbiogenesis, a new study of the origin of organisms [part 1 of 4]]. Biologisches Centralblatt (in German). 30 (8): 278–288.
- Mereschkowsky, Konstantin (1 May 1910). "Theorie der zwei Plasmaarten als Grundlage der Symbiogenesis, einer neuen Lehre von der Entstehung der Organismen" [Theory of two types of plasma as the basis of symbiogenesis, a new study of the origin of organisms [part 2 of 4]]. Biologisches Centralblatt (in German). 30 (9): 289–303.
- Mereschkowski, Konstantin (15 May 1910). "Theorie der zwei Plasmaarten als Grundlage der Symbiogenesis, einer neuen Lehre von der Entstehung der Organismen" [Theory of two types of plasma as the basis of symbiogenesis, a new study of the origin of organisms [part 3 of 4]]. Biologisches Centralblatt (in German). 30 (10): 321–347.
- Mereschkowsky, Konstantin (1 June 1910). "Theorie der zwei Plasmaarten als Grundlage der Symbiogenesis, einer neuen Lehre von der Entstehung der Organismen" [Theory of two types of plasma as the basis of symbiogenesis, a new study of the origin of organisms [part 4 of 4]]. Biologisches Centralblatt (in German). 30 (11): 353–367.
- ↑ Martin, William; Mayo Roettger; Thorsten Kloesges; Thorsten Thiergart; Christian Woehle; Sven Gould; Tal Dagan. "Modern endosymbiotic theory: Getting lateral gene transfer in-to the equation" (PDF). Journal of Endocytobiosis and Cell Research. 23: 1–5. (journal URL: )
- ↑ See:
- Schimper, A. F. W. (16 February 1883). "Ueber die Entwicklung der Chlorophyllkörner und Farbkörper" [On the development of chlorophyll granules and colored bodies [part 1 of 4]]. Botanische Zeitung (in German). 41 (7): 105–114. From p. 105: "Inzwischen theilte mir Herr Professor Schmitz mit, dass … die höheren Pflanzen sich ebenso verhalten würden." (Meanwhile, Prof. Schmitz reported to me that among algae, the creation of chlorophyll granules from the cell plasma doesn't occur, but that they arise exclusively from one another by division. The spores receive from the mother plant chlorophyll granules, which create, by division, all of the chlorophyll granules of the plants that arises from them [i.e., the spores]. This finding in algae made it seem likely to Prof. Schmitz that the higher plants would behave likewise.) From p. 106: "Meine Untersuchungen haben ergeben, … aus dem Scheitelmeristem sich entwickelnden Gewebe erzeugen." (My investigations have revealed that the vegetation points [i.e., points of vegetative growth] always contain differentiated chlorophyll bodies or their colorless rudiments; that they arise not by creation from the cell plasma, but from one another by division, and that they create all chlorophyll bodies and starch-forming [bodies] of the tissues developing from the apical meristem.) From p. 112, footnote 2: "Sollte es sich definitiv bestätigen, … an eine Symbiose erinnern." (If it should definitely be confirmed that the plastids in egg cells are not formed anew, then their relation to the organism containing them would somewhat suggest a symbiosis.)
- Schimper, A. F. W. (23 February 1883). "Ueber die Entwicklung der Chlorophyllkörner und Farbkörper" [On the development of chlorophyll granules and colored bodies [part 2 of 4]]. Botanische Zeitung (in German). 41 (8): 121–131.
- Schimper, A. F. W. (2 March 1883). "Ueber die Entwicklung der Chlorophyllkörner und Farbkörper" [On the development of chlorophyll granules and colored bodies [part 3 of 4]]. Botanische Zeitung (in German). 41 (9): 137–146.
- Schimper, A. F. W. (9 March 1883). "Ueber die Entwicklung der Chlorophyllkörner und Farbkörper" [On the development of chlorophyll granules and colored bodies [part 4 of 4]]. Botanische Zeitung (in German). 41 (10): 153–162.
- ↑ Portier, Paul (1918). Les Symbiotes (in French). Paris, France: Masson et Cie. p. 293. From p. 293: "Cette modification dans les rapports des appareils nucléaire et mitochondrial peut être le résultat de deux mécanismes. … Cette la parthénogénèse." (This modification in the relations of the nuclear and mitochondrial systems could be the result of two mechanisms: (a) There is a combination of two factors: contribution of new symbionts by the spermatozoid and reduction division. That is fertilization. (b) A single factor exists: reduction division: in this case, the egg contains sufficiently active symbionts. That is parthenogenesis.)
- ↑ Lane, Nick (2005). Power, Sex, Suicide. Mitochondria and the Meaning of Life. New York: Oxford University Press. p. 14. ISBN 9780199205646.
- ↑ Wallin, I. E. (1923). "The Mitochondria Problem". The American Naturalist. 57 (650): 255–61. doi:10.1086/279919.
- ↑ Wallin, I. E. (1927). Symbionticism and the origin of species. Baltimore: Williams & Wilkins Company. p. 117.
- ↑ Margulis, Lynn (2011). "Symbiogenesis. A new principle of evolution rediscovery of Boris Mikhaylovich Kozo-Polyansky (1890–1957)". Paleontological Journal. 44 (12): 1525–1539. doi:10.1134/S0031030110120087.
- ↑ Niklas, Karl J. (2010). "Boris M. Kozo-Polyansky, Symbiogenesis: A New Principle of Evolution". Symbiosis. 52 (1): 49–50. doi:10.1007/s13199-010-0098-7.
- ↑ Corning, Peter A. (2010). Holistic Darwinism: Synergy, Cybernetics, and the Bioeconomics of Evolution. Chicago: University of Chicago Press. p. 81. ISBN 978-0-22611-633-4.
- ↑ Ris, H.; Singh, R. N. (January 1961). "Electron microscope studies on blue-green algae". J Biophys Biochem Cytol. 9 (1): 63–80. doi:10.1083/jcb.9.1.63. PMC 2224983. PMID 13741827.
- ↑ Stocking, C.; Gifford, E. (1959). "Incorporation of thymidine into chloroplasts of Spirogyra". Biochem. Biophys. Res. Commun. 1 (3): 159–64. doi:10.1016/0006-291X(59)90010-5.
- ↑ Sagan, Lynn (1967). "On the origin of mitosing cells". J Theor Biol. 14 (3): 255–274. doi:10.1016/0022-5193(67)90079-3. PMID 11541392.
- ↑ Margulis, Lynn; Sagan, Dorion (2001). "Marvellous microbes". Resurgence. 206: 10–12.
- ↑ Gabaldón, T.; Snel, B.; van Zimmeren, F.; Hemrika, W.; Tabak, H.; Huynen, M.A. (2006). "Origin and evolution of the peroxisomal proteome". Biol. Direct. 1 (1): 8. doi:10.1186/1745-6150-1-8. PMC 1472686. PMID 16556314. (Provides evidence that contradicts an endosymbiotic origin of peroxisomes. Instead it is suggested that they evolutionarily originate from the endoplasmic reticulum)
- 1 2 Keeling, P. J.; Archibald, J.M. (2008). "Organelle evolution: what's in a name?". Current Biology. 18: 345–347. doi:10.1016/j.cub.2008.02.065. PMID 18430636.
- ↑ Michael Syvanen, Clarence I. Kado Horizontal Gene Transfer Academic Press, p. 405 ISBN 978-0126801262
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Timmis, Jeremy N.; Ayliffe, Michael A.; Huang, Chun Y.; Martin, William (2004). "Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes". Nature Reviews Genetics. 5 (2): 123–135. doi:10.1038/nrg1271. PMID 14735123.
- 1 2 3 4 5 6 Lila Koumandou, V.; Nisbet, R. Ellen R.; Barbrook, Adrian C.; Howe, Christopher J. (2004). "Dinoflagellate chloroplasts – where have all the genes gone?". Trends in Genetics. 20 (5): 261–267. doi:10.1016/j.tig.2004.03.008. PMID 15109781.
- ↑ Taanman, Jan-Willem (1999-02-09). "The mitochondrial genome: structure, transcription, translation and replication". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1410 (2): 103–123. doi:10.1016/S0005-2728(98)00161-3.
- ↑ Nowack, E.C.; Melkonian, M.; Glockner, G. (2008). "Chromatophore genome sequence of Paulinella sheds light on acquisition of photosynthesis by eukaryotes". Current Biology. 18: 410–418. doi:10.1016/j.cub.2008.02.051. PMID 18356055.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Barbrook, Adrian C.; Howe, Christopher J.; Purton, Saul (2006). "Why are plastid genomes retained in non-photosynthetic organisms?". Trends in Plant Science. 11 (2): 101–108. doi:10.1016/j.tplants.2005.12.004. PMID 16406301.
- 1 2 3 4 5 6 Leister, Dario (2005). "Origin, evolution and genetic effects of nuclear insertions of organelle DNA". Trends in Genetics. 21 (12): 655–663. doi:10.1016/j.tig.2005.09.004. PMID 16216380.
- ↑ Keeling, Patrick J. (2004). "Diversity and evolutionary history of plastids and their hosts". American Journal of Botany. 91 (10): 1481–1493. doi:10.3732/ajb.91.10.1481. PMID 21652304.
- 1 2 3 Archibald, John M. (January 2009). "The Puzzle of Plastid Evolution". Current Biology. 19 (2): R81–R88. doi:10.1016/j.cub.2008.11.067. PMID 19174147.
- ↑ "Two new studies confirm the 'endogenosymbiosis' hypothesis". Retrieved 2016-12-05.
- ↑ Cazzolla Gatti, Roberto (2016). "A conceptual model of new hypothesis on the evolution of biodiversity". Biologia. 71 (3). doi:10.1515/biolog-2016-0032.
- ↑ "Mitochondria Share an Ancestor With SAR11, a Globally Significant Marine Microbe". ScienceDaily. July 25, 2011. Retrieved 2011-07-26.
- ↑ Thrash, J. Cameron; et al. (2011). "Phylogenomic evidence for a common ancestor of mitochondria and the SAR11 clade". Scientific Reports. 1: 13. doi:10.1038/srep00013. PMC 3216501. PMID 22355532.
- ↑ Deusch, O.; et al. (2008). "Genes of cyanobacterial origin in plant nuclear genomes point to a heterocyst-forming plastid ancestor". Mol. Biol. Evol. 25: 748–761. doi:10.1093/molbev/msn022.
- ↑ Ochoa de Alda, J. A. G.; Esteban, R.; Diago, M. L.; Houmard, J. (2014). "The plastid ancestor originated among one of the major cyanobacterial lineages". Nature Communications. 5: 4937. doi:10.1038/ncomms5937. PMID 25222494.
- ↑ Howe, Christopher J. (2008). "Cellular Evolution: What's in a Mitochondrion?". Current Biology. 18 (10): R429–R431. doi:10.1016/j.cub.2008.04.007. PMID 18492476.
- 1 2 3 Lane, Nick (2011). "Plastids, Genomes, and the Probability of Gene Transfer". Genome Biology and Evolution. 3: 372–374. doi:10.1093/gbe/evr003. PMC 3101016. PMID 21292628.
- ↑ Kimball, J. 2010. Kimball's Biology Pages. Accessed October 13, 2010. An online open source biology text by Harvard professor, and author of a general biology text, John W. Kimball.
- ↑ Reece, J., Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson, 2010. Campbell Biology. 9th Edition Benjamin Cummings; 9th Ed. (October 7, 2010)
- ↑ Raven, P., George Johnson, Kenneth Mason, Jonathan Losos, Susan Singer, 2010. Biology. McGraw-Hill 9th Ed. (January 14, 2010)
- ↑ Gray, MW (1992). "The endosymbiont hypothesis revisited". International Review of Cytology. 141: 233–357. doi:10.1016/S0074-7696(08)62068-9.
- ↑ Zimorski, V.; Ku, C.; Martin, W. F.; Gould, S. B. (2014). "Endosymbiotic theory for organelle origins". Curr Opin Microbiol. 22: 38–48. doi:10.1016/j.mib.2014.09.008. PMID 25306530.
- ↑ Margolin, William (November 2005). "FtsZ and the Division of Prokaryotic Cells and Organelles". Nat Rev Mol Cell Biol. 6 (11): 862–871. doi:10.1038/nrm1745. PMC 4757588. PMID 16227976.
- ↑ Wise, Robert R; Hoober, J. Kenneth (2007). Structure and function of plastids. Berlin: Springer. p. 104. ISBN 9781402065705.
- ↑ Fischer, K, Weber, A, Brink, S, Arbinger, B, Schünemann, D, Borchert, S, Heldt, HW, Popp, B, Benz, R, Link, TA (1994). "Porins from plants. Molecular cloning and functional characterization of two new members of the porin family". J Biol Chem. 269 (41): 25754–25760. PMID 7523392.
- ↑ Zeth K.; Thein, M. (2010). "Porins in prokaryotes and eukaryotes: common themes and variations". Biochem J. 431 (1): 13–22. doi:10.1042/BJ20100371. PMID 20836765.
- ↑ Fairman, JW; Noinaj, N; Buchanan, SK (2011). "The structural biology of β-barrel membrane proteins: a summary of recent reports". Current Opinion in Structural Biology. 21 (4): 523–531. doi:10.1016/j.sbi.2011.05.005. PMC 3164749. PMID 21719274.
- ↑ Mileykovskaya, E.; Dowhan, W. (2009). "Cardiolipin membrane domains in prokaryotes and eukaryotes". Biochim Biophys Acta. 1788 (10): 2084–2091. doi:10.1016/j.bbamem.2009.04.003. PMC 2757463. PMID 19371718.
- 1 2 Timmis, Jeremy; Ayliffe, Michael; Huang, Chun; Martin, William (February 2004). "Endosymbiotic Gene Transfer: Organelle Genomes Forge Eukaryotic Chromosomes". Nature Reviews Genetics. 5: 123–135. doi:10.1038/nrg1271. PMID 14735123.
- ↑ Andersson, SG, Zomorodipour, A, Andersson, JO, Sicheritz-Pontén, T, Alsmark, UC, Podowski, RM, Näslund, AK, Eriksson, AS, Winkler, HH, Kurland, CG (1998). "The genome sequence of Rickettsia prowazekii and the origin of mitochondria". Nature. 396 (6707): 133–140. doi:10.1038/24094. PMID 9823893.
- ↑ Dagan, T, Roettger, M, Stucken, K, Landan, G, Koch, R, Major, P, Gould, SB, Goremykin, VV, Rippka, R, Tandeau de Marsac, N, Gugger, M, Lockhart, PJ, Allen, JF, Brune, I, Maus, I, Pühler, A, Martin, WF (2013). "Genomes of Stigonematalean cyanobacteria (subsection V) and the evolution of oxygenic photosynthesis from prokaryotes to plastids". Genome Biol Evol. 5 (1): 31–44. doi:10.1093/gbe/evs117. PMC 3595030. PMID 23221676.
- ↑ Manuell, Andrea L.; Quispe, Joel; Mayfield, Stephen P. (August 2007). "Structure of the Chloroplast Ribosome: Novel Domains for Translation Regulation". PLOS Biology. 5: e209. doi:10.1371/journal.pbio.0050209. PMC 1939882. PMID 17683199.
- ↑ Schwartz, James; Meyer, Ralph; Eisenstadt, Jerome; Brawerman, George (1967). "Involvement of N-formylmethionine in initiation of protein synthesis in cell-free extracts of Euglena gracilis". J Mol Biol. 25 (3): 571–IN27. doi:10.1016/0022-2836(67)90210-0. Retrieved 18 May 2016.
- ↑ Smith, A. E.; Marcker, K. A. (1968). "N-formylmethionyl transfer RNA in mitochondria from yeast and rat liver". J Mol Biol. 38 (2): 241–243. doi:10.1016/0022-2836(68)90409-9. Retrieved 18 May 2016.
- ↑ McFadden, G. I. (2001). "Primary and secondary endosymbiosis and the origin of plastids". J Phycol. 37 (6): 951–9. doi:10.1046/j.1529-8817.2001.01126.x.
- ↑ Okamoto, Noriko; Inouye, Isao (2005). "A Secondary Symbiosis in Progress?" (PDF). Science. 310 (5746): 287. doi:10.1126/science.1116125. PMID 16224014. Retrieved 15 February 2015.
- ↑ McFadden, G. I.; van Dooren, G. G. (July 2004). "Evolution: red algal genome affirms a common origin of all plastids". Curr. Biol. 14 (13): R514–6. doi:10.1016/j.cub.2004.06.041. PMID 15242632.
- ↑ Gould, SB, Waller, RF, McFadden, GI (2008). "Plastid evolution". Annu Rev Plant Biol. 59 (1): 491–517. doi:10.1146/annurev.arplant.59.032607.092915. PMID 18315522.
- ↑ Georgiades, K.; Raoult, D. (2011). "The rhizome of Reclinomonas americana, Homo sapiens, Pediculus humanus and Saccharomyces cerevisiae mitochondria". Biology Direct. 6: 55. doi:10.1186/1745-6150-6-55.
- ↑ Prasad, Pijai (August 2005). "Organic-walled microfossils from the Proterozoic Vindhyan Supergroup of Son Valley, Madhya Pradesh, India" (PDF). Paleobotanist. 54.
- ↑ Butterfield, Nicholas J. (2014-11-26). "Early evolution of the Eukaryota". Palaeontology. 58 (1): 5–17. doi:10.1111/pala.12139.
- ↑ Parfrey, Laura Wegener; Lahr, Daniel J. G.; Knoll, Andrew H.; Katz, Laura A. (2011-08-16). "Estimating the timing of early eukaryotic diversification with multigene molecular clocks". Proceedings of the National Academy of Sciences. 108 (33): 13624–13629. doi:10.1073/pnas.1110633108. PMC 3158185. PMID 21810989.
- ↑ Hedges, S. Blair; Blair, Jaime E.; Venturi, Maria L.; Shoe, Jason L. (2004-01-28). "A molecular timescale of eukaryote evolution and the rise of complex multicellular life". BMC Evolutionary Biology. 4: 2. doi:10.1186/1471-2148-4-2. PMC 341452. PMID 15005799.
- ↑ Gross, Jeferson; Bhattacharya, Debashish (2010-08-23). "Uniting sex and eukaryote origins in an emerging oxygenic world". Biology Direct. 5: 53. doi:10.1186/1745-6150-5-53. PMC 2933680. PMID 20731852.
- ↑ Butterfield, Nicholas J. (1997/ed). "Plankton ecology and the Proterozoic-Phanerozoic transition". Paleobiology. 23 (2): 247–262. doi:10.1017/S009483730001681X. Check date values in:
|date=(help)
Further reading
- Alberts, Bruce (2002). Molecular Biology of the Cell. New York: Garland Science. ISBN 0-8153-3218-1. (General textbook)
- Brinkman, F. S., Blanchard, J. L., Cherkasov A, et al. (August 2002). "Evidence that plant-like genes in Chlamydia species reflect an ancestral relationship between Chlamydiaceae, cyanobacteria, and the chloroplast". Genome Res. 12 (8): 1159–67. doi:10.1101/gr.341802. PMC 186644. PMID 12176923.
- Cohen, W. D.; Gardner, R. S. (1959). "Viral Theory and Endosymbiosis" (PDF). (Discusses theory of origin of eukaryotic cells by incorporating mitochondria and chloroplasts into anaerobic cells with emphasis on 'phage bacterial and putative viral mitochondrial/chloroplast interactions.)
- Jarvis, P. (April 2001). "Intracellular signalling: the chloroplast talks!". Curr. Biol. 11 (8): R307–10. doi:10.1016/S0960-9822(01)00171-3. PMID 11369220. (Recounts evidence that chloroplast-encoded proteins affect transcription of nuclear genes, as opposed to the more well-documented cases of nuclear-encoded proteins that affect mitochondria or chloroplasts.)
- Blanchard, J. L.; Lynch, M. (July 2000). "Organellar genes: why do they end up in the nucleus?". Trends Genet. 16 (7): 315–20. doi:10.1016/S0168-9525(00)02053-9. PMID 10858662. (Discusses theories on how mitochondria and chloroplast genes are transferred into the nucleus, and also what steps a gene needs to go through in order to complete this process.)
- Okamoto, N.; Inouye, I. (October 2005). "A secondary symbiosis in progress?". Science. 310 (5746): 287. doi:10.1126/science.1116125. PMID 16224014.
- Understanding Science Team. "Cells within cells: An extraordinary claim with extraordinary evidence" (PDF). University of California, Berkeley. Retrieved 16 February 2014.