Reversine
 | |
| Names | |
|---|---|
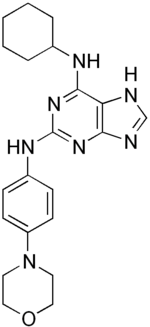
| IUPAC name
N'-cyclohexyl-N-(4-morpholinophenyl)-7H-purine-2,6-diamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.164.070 |
| MeSH | C484369 |
PubChem CID |
|
| |
| |
| Properties | |
| C21H27N7O | |
| Molar mass | 393.48538 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reversine, or 2-(4-morpholinoanilino)-6-cyclohexylaminopurine, is a small molecule developed by the group of Peter G. Schultz, used for stem cell dedifferentiation.
It also has the potential to induce selectively cell death of cancer cells.
Reversine is known to act as an antagonist of the adenosine A3 receptor. Reversine is a potent inhibitor of the mitotic kinase Mps1[1] and it is widely used to study the process of chromosome segregation.
References
- ↑ Santaguida, Stefano; Tighe, Anthony; D'Alise, Anna Morena; Taylor, Stephen S.; Musacchio, Andrea (2010-07-12). "Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine". The Journal of Cell Biology. 190 (1): 73–87. doi:10.1083/jcb.201001036. ISSN 1540-8140. PMC 2911657. PMID 20624901.
- Chen, Shuibing; Zhang, Qisheng; Wu, Xu; Schultz, Peter G.; Ding, Sheng (2004). "Dedifferentiation of Lineage-Committed Cells by a Small Molecule". Journal of the American Chemical Society. 126 (2): 410–1. doi:10.1021/ja037390k. PMID 14719906.
- Chen, S.; Takanashi, S.; Zhang, Q.; Xiong, W.; Zhu, S.; Peters, E. C.; Ding, S.; Schultz, P. G. (2007). "Reversine increases the plasticity of lineage-committed mammalian cells". Proceedings of the National Academy of Sciences. 104 (25): 10482–7. Bibcode:2007PNAS..10410482C. doi:10.1073/pnas.0704360104. PMC 1965539. PMID 17566101.
- Henry, Celia (January 5, 2004). "Cellular U-Turn". Chemical and Engineering News. 82 (1): 9. doi:10.1021/cen-v082n001.p009.
- Piccoli, Marco; Palazzolo, Giacomo; Conforti, Erika; Lamorte, Giuseppe; Papini, Nadia; Creo, Pasquale; Fania, Chiara; Scaringi, Raffaella; Bergante, Sonia; Tringali, Cristina; Roncoroni, Leda; Mazzoleni, Stefania; Doneda, Luisa; Galli, Rossella; Venerando, Bruno; Tettamanti, Guido; Gelfi, Cecilia; Anastasia, Luigi (2012). "The synthetic purine reversine selectively induces cell death of cancer cells". Journal of Cellular Biochemistry. 113 (10): 3207–17. doi:10.1002/jcb.24197. PMID 22615034.
- Santaguida, S; Tighe, A; D'Alise, AM; Taylor, SS; Musacchio, A (Jul 12, 2010). "Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine.". The Journal of Cell Biology. 190 (1): 73–87. doi:10.1083/jcb.201001036. PMID 20624901
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.