Pantoprazole
 | |
| Clinical data | |
|---|---|
| Trade names | originally Protonix, subsequently many others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601246 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth and intravenous |
| Drug class | proton pump inhibitor |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 77% |
| Metabolism | Liver (CYP2C19) |
| Elimination half-life | 1-2 hours |
| Excretion | Urine, Feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.111.005 |
| Chemical and physical data | |
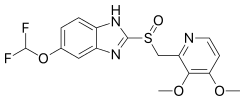
| Formula | C16H15F2N3O4S |
| Molar mass | 383.371 g/mol |
| 3D model (JSmol) | |
| Chirality | Racemic |
| |
| |
| (verify) | |
Pantoprazole, first sold under the brand name Protonix, is used for short-term treatment of erosive esophagitis associated with gastroesophageal reflux disease (GERD), maintenance of healing of erosive esophagitis, and pathological hypersecretory conditions including Zollinger–Ellison syndrome.[2]
Some common side effects of pantoprazole use in adults include: headache, diarrhea, nausea, abdominal pain, vomiting, flatulence, dizziness, and joint pain (>2%).[2] Use of pantoprazole for a long period of time may lead to chronic inflammation of stomach lining or atrophic gastritis, vitamin B-12 deficiency, and low magnesium.[2][3]
Pantoprazole is a proton pump inhibitor drug that inhibits gastric acid secretion. It works on gastric parietal cells to irreversibly inhibit (H+/K+)-ATPase function and suppress the production of gastric acid.[2][4] It was first sold in 1994 in Germany and became available as a generic medication in 2010.[1]
Medical uses
Pantoprazole is used for short-term treatment of erosion and ulceration of the esophagus for adults and pediatric patients 5 years of age and older caused by gastroesophageal reflux disease.[2] It can be used as a maintenance therapy for long-term use after initial response is obtained, but there have not been any controlled studies about the use of pantoprazole past a duration of 12 months.[2] Pantoprazole may also be used in combination with antibiotics to treat ulcers caused by Helicobacter pylori.[5] It can also be used for long-term treatment of Zollinger-Ellison syndrome.[2]
Pregnancy
U.S.A Pregnancy Category B: In reproductive studies using doses largely greater than the recommended doses performed on rats and rabbits, there was no evident harm on the development of the baby.[2]
Breast feeding
Pantoprazole has been found to pass through the breast milk. However, in rodent cancer studies, pantoprazole has been shown to potentially cause tumor growth. The clinical relevance of the finding is unknown, but risks and benefits are recommended for consideration in determining the use of therapy for the mother and child.[2]
Children
Pantoprazole is only indicated for the short-term treatment of erosive esophagitis in children ages 7 and older; and the safety and effectiveness of pantoprazole have only been established in the treatment of erosive esophagitis in children.[2]
Elderly
The incidence of adverse effects occurring in patients aged 65 years and older were similar to those in patients aged 65 years and less.[2]
Adverse effects
- Infection: Stomach acid plays a role in killing ingested bacteria. Use of pantoprazole may increase the chance of developing infections such as pneumonia, particularly in hospitalized patients.[6]
Common
- Gastrointestinal: abdominal pain (6%), diarrhea (9%), flatulence (4%), nausea (7%), vomiting (4%)[2]
- Neurologic: headache (12%), dizziness (3%)[2]
- Neuromuscular and skeletal: arthralgia (3%)[2]
Rare
- Gastrointestinal: constipation, dry mouth, hepatitis[2]
- Blood problems: low white blood cell count, thrombocytopenia[2]
- Immunologic: Stevens-Johnson syndrome, toxic epidermal necrolysis[2]
- Metabolic: elevated creatine kinase, elevated cholesterol levels, elevated liver enzymes (AST/ALT), swelling[2]
- Musculoskeletal: Muscle disorders, bone fracture and infection, Clostridium difficile infection, osteoporosis-related hip fracture, rhabdomyolysis[2]
- Kidneys: interstitial nephritis[7]
- Nutrition: may reduce the absorption of important nutrients, vitamins, and minerals, including certain medications, leaving users at increased risk for pneumonia.[8]
Long-term use
- Osteoporosis and bone fracture have been observed in patients on high-dose and/or long term (over 1 year) prescription proton pump inhibitors.[9]
- Hypomagnesia has been observed in patients on medications like pantoprazole when taken for longer periods of time (generally 1 year or more, although cases have been reported with regimens as short as 3 months).[3]
Discontinuation
In people on PPIs for longer than six months, a dose taper should be considered prior to discontinuation. For those on a moderate- to high-dose this can be done by 50 percent every week until on the lowest dose. After a week it can then be stopped.[10]
Interactions
- Acidity: Due to its effect of reducing stomach acidity, use of pantoprazole can affect absorption of drugs that are pH-sensitive such as ampicillin esters, ketoconazole, atazanavir, iron salts, amphetamine and mycophenolate mofetil.[2]
Pharmacology

The mechanism of action of pantoprazole is to inhibit the final step in gastric acid production.[2] In the gastric parietal cell of the stomach, pantoprazole covalently binds to the H+/K+ ATP pump to inhibit gastric acid and basal acid secretion.[2] The covalent binding prevents acid secretion of up to 24 hours and longer.[2]
Pantoprazole is metabolized in the liver by the cytochrome P450 system.[11] Metabolism mainly consists of demethylation by CYP2C19 followed by sulfation. Another metabolic pathway is oxidation by CYP3A4. Pantoprazole metabolites are not thought to have any pharmacological significance. It is usually given with a prokinetic drug because of inactivity in the acidic environment of the stomach. Pantoprazole binds irreversibly to H+K+ATPase (proton pumps) to suppress the secretion of acid. Due to irreversible binding of the pumps, new pumps have to be made before acid production can be resumed.[4] The drug's plasma half-life is about 2 hours.[12]
History
Pantoprazole was discovered by scientists at Byk Gulden, a subsidiary of Altana; the drug discovery program started in 1980 and which produced pantoprazole in 1985 - the compound was actually created by chemists working on scaleup of a different chemical that had been chosen as a development candidate.[13]:117,129 Byk Gulden partnered with Smith Kline & French in 1984.[13]:124 The compound's development names were BY1029 and SK&F96022.[13]:123 By 1986 the companies had created the sodium salt, pantoprazole sodium sesquihydrate, and decided to develop it as it was more soluble and stable, and was more compatible with other ingredients used in the formulation.[13]:130 It was first marketed in Germany in 1994.[13]:130 Wyeth licensed the US patent from Altana.[14] and obtained marketing approval from the US FDA in 2000 under the trade name Protonix.[15]
In 2004 worldwide sales of the drug were $3.65 billion, about half of which were in the US.[14]
In 2007 Altana's drug business was acquired by Nycomed.[16] Nycomed was in turn acquired by Takeda in 2011[17] and Wyeth was acquired by Pfizer in 2009.[18]
The patent protecting the drug was set to expire in 2010, but Teva Pharmaceutical filed an ANDA in 2007, and Wyeth and Nycomed sued Teva for patent infringement, but Teva decided to launch its generic drug "at risk" that year, before the patent had been invalidated.[19][20] Wyeth launched an authorized generic in 2008.[16] Pfizer and Takeda's patent exclusivity expired in 2010, and an administrative exclusivity they had for pediatric use expired in January 2011, and full generic competition began.[21] The litigation between Teva and Pfizer/Takeda was settled in 2013, with Teva paying the patent holders $2.15 billion in damages for its early launch.[22]
Society and culture
As of 2017, the drug was marketed under many brands worldwide, including as a combination drug with domperidone, a combination with itopride, in combination with both clarithromycin and amoxicillin, in combination with levosulpiride, and in combination with naproxen.[1]
| List of brand names |
|---|
|
As of 2017, was marketed under many brands worldwide, including: Acernix, Aciban, Acida, Acido-X, Acidrol, Acidwell, Acilib, Acilibre, Acillect, Acipan, Acrid, Alapanzol, Amphoter, Anagastra, Anesteloc, Antaxid, Antopral, Anulacid, Anxel, Apazol, Appryo, Aptizole, Apton, Armcid, Asoprazole, Aspan, Aurizol-P, Awamed, Azatol, Biotop V, Brandocare, Branzol, Buffet, Buscopan Reflusso, Caprol, Ciprazol, Citrel, Clessol, Comenazol, Conoran, Contix, Contracid, Contraflux, Contro-Cap, Controloc, Controloc, Cool Pan, Delpanto EC, Digene Total, Digespan, Dosanloc, Empaflun, Eracid, Erprazol, Esopan, Eupantol, Exopan, Extream, Extreme, F-Pan, Farmazol, Fenix, Fexmor, Fu Shi Tan, Fulpan, Fupan, Gastblok, Gastenz, Gastrazol-L, Gastriwin, Gastrolan, Gastroloc, Gastromax, Gastronorm, Gastroprozal, Gastrostad, Gastrowell, Gastrozol, Gerdamegh, Gerprazol, Gesoflux, Gondea, Gopan, Hansazol, Hasanloc, Helix, Iboprot, Inipant, Inipepsia, Inipomp, IPP, Ippracid, Ipraalox, Kaiji, Kairol, Letopra, Loxanto, Luoxu, Lupipan, Maalox, Mag, Manez, Marozel, Monpan, Nelgast, Nexpan, Noacid, Noacid, Nolpaza, Nolpaza, Normogastrol, Noxadif, Ntap, Nuosen, Nupenta, Oritop, Osipan, Ozepran, Ozpan, Ozzion, P-20, P-40, P-Bit, P-OD, P-PPI, P-Zole, Pacid, Paciddia, Palio, Palmy, Pamel, Pamtrazol, Pamyl, Pan, Panbloc, Pancleus, Pancrazio, Pandev, Pane, Panfast, Pangest, Panglen, Panlan, Panlisu, Panloc, Panloz, Panmeilu, Panocer, Panogastin, Panopaz, Panor, Panoral, Panore, Panpot, Panpra, Panprabene, Panprax, Panprazol, Panprazox, Panpro, Panproton, Panpure, Panrazol, Panrazole, Panrbe, Panref, Pansa, Pansec, Panso, Pantac, Pantacid, Pantact, Pantagi, Pantakind, Pantaltius, Pantap, Pantasur, Pantaz, Pantazol, Pantecta, Pantex, Pantexel, Pantezol, Panthec, Panthron, Pantid, Pantin, Pantip, Pantium, Panto, Panto-Denk, Panto-Gas, Pantobex, Pantoc, Pantocal, Pantocar, Pantocare, Pantocas, Pantocer, Pantocid, Pantocim, Pantocom, Pantocure, Pantodac, Pantodar, Pantofin, Pantofir, Pantogastrix, Pantogen, Pantogerolan, PantoJenson, Pantokem, Pantokool, Pantolax, Pantoline, Pantoloc, Pantolok, Pantolup, Pantomax, Pantomed, Pantometylentina, Pantomyl, Pantonis, Pantonix, Pantop, Pantopacid, Pantopan, Pantopaz, Pantopep, Pantopi, Pantopra-Q, Pantopraz, Pantoprazal, Pantoprazol, Pantoprazole, Pantoprazolo, Pantoprazolum, Pantoprem, Pantoprix, Pantoprol, Pantopump, Pantor, Pantorc, Pantoren, Pantorica, Pantosal, Pantosan, Pantosec, Pantosid, Pantostad, Pantotab, Pantotis, Pantover, Pantoz, Pantozim, Pantozol, Pantozole, Pantpas, Pantra, Pantrol, Pantroz, Pantul, Pantune, Pantus, Panveda, Panvell, Panz, Panzat, Panzel, Panzilan, Panzilan, Panzol, Panzole, Panzor, Parastamic, Paz, Peblo, Penkool, Penlip, Pentalink, Pentastar, Pentowin, Pentoz, Pentozed, Peploc, Peptac, Peptazol, Peptazole, Pepticaid, Pepticool, Peptix, Peptoloc, Pepzol, Perloc, Pipanzin, Pozola, Praize, Pranza, Praz-Up, Prazobloc, Prazocid, Prazolacid, Prazolan, Prazole, Prazolpan, Prazopant, Pregel, Prevacid, Previfect, Previfect, Progen, Prolex, Promtec, Propanz, Protech, Protinum, Protium, Protocent, Protocid, Protofix, Protoloc, Proton, Proton-P, Protonex, Protonil, Protonix, Protopan, PTA, Pulcet, Pumpisel, Ranloc, Razon, Rcpan, Redacib, Refluxine, Refluxopan, rifun, Ripane, Roxitrol, Sedipanto, Segregam, Seltraz, Sipar, Sodac, Somac, Sozol, Stamic, Stomafor, Stripole, Sumipral, Supacid, Super OM, Suppi, Supracam, Supracid, Surmera, Tai Mei Ni Ke, Tecta, Tonval, Topazol, Topra, Topraz, Topzole, Toraflux, Tropaz, Trupan, Ulceron, Ulcoreks, Ulcotenal, Ulprix, Ulsepan, Ulstop, Ultop, Ultoz, Unigastrozol, Vencid, Ventro-Pant, Vomizole, Wei Di, Wei Ke An, Wonon, Xotepic, Yoevid, Zamotil, Zaprol, Zencopan, Zgaton, Zimpax, Zipant, Zipantol, Zipantola, Ziprol, Zolan, Zolemer, Zolpan, Zolpanz, Zolpra, Zoltex, Zoltum, Zontop, Zoprax, Zovanta, Zurcal, and Zurcazol.[1] It was also marketed as a combination drug with domperidone under the brand names Aciban-DSR, Acillect-DSR, Asoprazole-D, Buffet-DXR, Depam, Domelong P, Dycizol, Eracid-D, F-Pan DSR, Fulpan-D, Fulpan-DSR, Gerdom, Gi-Fri, Gopan-D, Gopan-DSR, GR8-OD, Kurepane-DSR, Latop-D, Monpan-D, Monpan-DSR, Nupenta-DSR, Odipan-DSR, Oritop-D, Oritop-DSR, P-Bit-D, P-Bit-DSR, P-Zole DSR, P-Zole-D, PAA-DSR, Palio-D, Pamtrazol-D, Pan-D, Pancrazio-DSR, Pandiff, Pandostal, Pandostal-OD, Panfast-DSR, Panopaz-D, Panor-D, Panpot-DSR, Pansa-D, Pantact-D, Pantin-D, Pantin-RD, Pantocar-D, Pantocom-D, Pantoflux, Pantojoy-DXR, Pantokool-D, Pantolex-DS, Pantopacid-D, Pantopacid-SR, Pantorica-D, Pantozol-D, Pantozol-DSR, Pantra-D, Pantune-D, Panveda-D, Panzo-D, Panzol Plus, Panzol-D, Paz-DN, Peblo-D, Peblo-DSR, Penkool-DSR, Penlip-D, Pentalink-D, Pentastar-D, Pentozed-D, Peptac D, Peptac DSR, Pepticool-DXR, Pintel-DSR, Pop-DSR, Praize-D, Praize-D Forte, Prazole Plus, Prazosan-DSR, Predom, Predom-OD, Prolex-DSR, Prolus-DSR, Protocent-DSR, Protopan-D, Protopan-H, Ripane-D, Ripane-DSR, Trazol-DSR, PTA-D, Ulcicap-PD, Ultop DSR, Ultoz-D, Wonon-D, Wonon-DSR, and Zovanta-D.[1] It was also marketed in combination with itopride under the brand names Ganaton Total, Kurepan-IT, Nupenta-ITR, P-Bit-ISR, Pepnil-ITO, Prolus-ISR, and Protopan-I.[1] It was also marketed in combination with clarithromycin and amoxicillin as Gastrocomb, Klacid Hp7, Panclamox, and ZacPac.[1] It was also marketed in combination with levosulpiride as Panlife-LS and in combination with naproxen as Arthopan.[1] |
References
- 1 2 3 4 5 6 7 8 "Pantoprazole international brand names". Drugs.com. Retrieved 15 March 2017.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 "Prescribing Info". Protonix package insert. Retrieved November 3, 2015.
- 1 2 Research, Center for Drug Evaluation and. "Drug Safety and Availability - FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs)". www.fda.gov. Retrieved 2015-11-03.
- 1 2 Richardson, Paul; Hawkey, Christopher J.; Stack, Dr William A. (2012-11-29). "Proton Pump Inhibitors". Drugs. 56 (3): 307–335. doi:10.2165/00003495-199856030-00002. ISSN 0012-6667. PMID 9777309.
- ↑ Dammann, Hans-Gerd; Fölsch, Ulrich R.; Hahn, Eckhart G.; Von Kleist, Detlef-Hasso; Klör, Hans-Ulrich; Kirchner, Thomas; Strobel, Sonja; Kist, Manfred (2000-03-01). "Eradication of H. pylori with Pantoprazole, Clarithromycin, and Metronidazole in Duodenal Ulcer Patients: A Head-to-Head Comparison Between Two Regimens of Different Duration". Helicobacter. 5 (1): 41–51. doi:10.1046/j.1523-5378.2000.00006.x. ISSN 1523-5378. PMID 10672051.
- ↑ Herzig, SJ; Doughty, C; Lahoti, S; Marchina, S; Sanan, N; Feng, W; Kumar, S (November 2014). "Acid-suppressive medication use in acute stroke and hospital-acquired pneumonia". Annals of Neurology. 76 (5): 712–8. doi:10.1002/ana.24262. PMC 4214881. PMID 25164323.
- ↑ Ricketson, Jeffrey; Kimel, Gil; Spence, James; Weir, Rene (2009-03-03). "Acute allergic interstitial nephritis after use of pantoprazole". Canadian Medical Association Journal. 180 (5): 535–538. doi:10.1503/cmaj.080456. ISSN 0820-3946. PMC 2645468. PMID 19255077.
- ↑ [Dr. John Cooke, chair of Methodist Hospital's cardiovascular services] [Houston Chronicle Health Zone dated Thursday, July 11, 2013 chron.com/refluxmeds] (Journal: Circulation)
- ↑ Research, Center for Drug Evaluation and. "Postmarket Drug Safety Information for Patients and Providers - FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors". www.fda.gov. Retrieved 2015-11-03.
- ↑ Wolfe, MM; Sachs, G (February 2000). "Acid suppression: optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome". Gastroenterology. 118 (2 Suppl 1): S9–31. doi:10.1016/s0016-5085(00)70004-7. PMID 10868896.
- ↑ Meyer, U A (1996). "Metabolic interactions of the proton-pump inhibitors lansoprazole, omeprazole and pantoprazole with other drugs". European Journal of Gastroenterology & Hepatology. 8 (Suppl 1): S21–25. doi:10.1097/00042737-199610001-00005.
- ↑ Sachs, George; Shin, Jai Moo; Hunt, Richard (2010-10-06). "Novel Approaches to Inhibition of Gastric Acid Secretion". Current Gastroenterology Reports. 12 (6): 437–447. doi:10.1007/s11894-010-0149-5. ISSN 1522-8037. PMC 2974194. PMID 20924727.
- 1 2 3 4 5 Senn-Bilfinger, Jörg; Sturm, Ernst (2006). "6. The Development of a New Proton-Pump Inhibitor: The Case History of Pantoprazole". In Fischer, János; Ganellin, C. Robin. Analogue-based drug discovery. Weinheim: Wiley-VCH. pp. 115–136. ISBN 9783527608003.
- 1 2 Daly, Erin Marie (May 20, 2008). "Wyeth, Nycomed Take Aim At Sandoz Over Protonix". Law360.
- ↑ Mathews, S; Reid, A; Tian, C; Cai, Q (2010). "An update on the use of pantoprazole as a treatment for gastroesophageal reflux disease". Clinical and Experimental Gastroenterology. 3: 11–6. PMC 3108659. PMID 21694841.
- 1 2 Goldstein, Jacob (30 January 2008). "Generic Protonix and Wyeth as Takeover Bait". WSJ.
- ↑ "Takeda to Buy Nycomed for $13.7 Billion". DealBook. May 19, 2011.
- ↑ "Pfizer in talks to acquire Wyeth in $60 billion deal: WSJ". MarketWatch. Retrieved 2012-08-11.
- ↑ Saul, Stephanie (7 September 2007). "Wyeth Faces Generic Rival to a Heartburn Drug". The New York Times.
- ↑ Curtiss, FR (April 2008). "Perspectives on the "generic cliff"--pushing and falling". Journal of Managed Care Pharmacy. 14 (3): 318–21. doi:10.18553/jmcp.2008.14.3.318. PMID 18439056.
- ↑ "Protonix - Big Patent Expirations of 2010". FiercePharma. Retrieved 15 March 2017.
- ↑ Helfand, Carly (June 12, 2013). "Teva loses $2B gamble on generic of Pfizer's Protonix". FiercePharma.