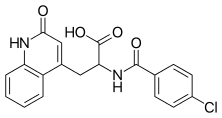
Rebamipide
 | |
| Clinical data | |
|---|---|
| Trade names | Mucosta (JP), Rebagen (KR, CN, IN), Rebagit (RU) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral (tablets) |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H15ClN2O4 |
| Molar mass | 370.786 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Rebamipide, an amino acid derivative of 2-(1H)-quinolinone, is used for mucosal protection, healing of gastroduodenal ulcers, and treatment of gastritis. It works by enhancing mucosal defense, scavenging free radicals, and temporarily activating genes encoding cyclooxygenase-2.
Rebamipide is used in a number of Asian countries including Japan (marketed as Mucosta), South Korea, China[1] and India (where it is marketed under the trade name Rebagen). It is also approved in Russia under the brand name Rebagit.[2] It is not approved by the Food and Drug Administration for use in the United States.
Studies have shown that rebamipide can fight the damaging effects of NSAIDs on the GIT mucosa, and more recently, the small intestine. It has also been studied for the treatment of Behçet's disease.[3] It was shown to successfully treat pouchitis in a single-N study after first-line therapies for the condition were unsuccessful.[4] Some studies have shown effectiveness in presbyacusis (age-related hearing loss).
It has also been shown to alleviate signs and symptoms of dry eyes in a randomised controlled trial although this is not yet widely available clinically.[5]
Articles
- Arakawa T, Watanabe T, Fukuda T, Yamasaki K, Kobayashi K (1995). "Rebamipide, novel prostaglandin-inducer accelerates healing and reduces relapse of acetic acid-induced rat gastric ulcer. Comparison with cimetidine". Dig Dis Sci. 40 (11): 2469–72. doi:10.1007/BF02063257. PMID 7587834.
- Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A (1998). "Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing". Dig Dis Sci. 43 (9 Suppl): 5S–13S. PMID 9753220.
- Tarnawski AS, Chai J, Pai R, Chiou SK (2004). "Rebamipide activates genes encoding angiogenic growth factors and Cox2 and stimulates angiogenesis: a key to its ulcer healing action?". Dig Dis Sci. 49 (2): 202–9. doi:10.1023/B:DDAS.0000017439.60943.5c. PMID 15104358.
- Takumida M, Anniko M (2009). "Radical scavengers for elderly patients with age-related hearing loss". Acta Otolaryngol. 129 (1): 36–44. doi:10.1080/00016480802008215. PMID 18607930.
References
- ↑ drugs.com
- ↑ "Russian State Register of Medicines. Registration Sertificate: Rebagit (rebamipide) Film-Coated Tablets" (in Russian). Retrieved 10 June 2017.
- ↑ Matsuda T, Ohno S, Hirohata S, Miyanaga Y, Ujihara H, Inaba G, Nakamura S, Tanaka S, Kogure M, Mizushima Y (2003). "Efficacy of rebamipide as adjunctive therapy in the treatment of recurrent oral aphthous ulcers in patients with Behcet's disease: a randomised, double-blind, placebo-controlled study". Drugs R D. 4 (1): 19–28. doi:10.2165/00126839-200304010-00002. PMID 12568631.
- ↑ http://www.wjgnet.com/1007-9327/12/656.pdf Archived October 20, 2013, at the Wayback Machine.
- ↑ Kinoshita, S.; K. Oshiden; S. Awamura; H. Suzuki; N. Nakamichi (2013). "A randomized, multicenter phase 3 study comparing 2% rebamipide (OPC-12759) with 0.1% sodium hyaluronate in the treatment of dry eye". Ophthalmology. 120 (6): 1158–65. doi:10.1016/j.ophtha.2012.12.022. PMID 23490326.