Demethylation
Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule.[1][2] A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen atoms.
The counterpart of demethylation is methylation.
In biochemistry
In biochemical systems, the process of demethylation is catalyzed by demethylases. These enzymes oxidize N-methyl groups, which occur in histones and some forms of DNA:
- R2N-CH3 + O → R2N-H + CH2O
One such oxidative enzyme family is the cytochrome P450.[3] Alpha-ketoglutarate-dependent hydroxylases are active for demethylation of DNA, operating by similar pathway. These reactions exploit the weak C-H bond adjacent to amines.
In organic chemistry
O-Demethylation
Demethylation typically refers to cleavage of ethers, especially aryl ethers, although there are some exceptions, for instance cf. "desipramine".
Aryl methyl ethers are pervasive in lignin and many derived compounds.[4] The demethylation of these materials has been the subject of much effort. The reaction typically requires harsh conditions or harsh reagents. For example, the methyl ether in vanillin can be removed by heating near 250 °C with strong base.[5] Stronger nucleophiles such as diorganophosphides (LiPPh2) also cleave aryl ethers under milder conditions.[6] A classical (but again, harsh) method for the removal of the methyl group of an aryl methyl ether is to reflux the ether in acetic acid and concentrated hydrobromic or hydroiodic acid.[7] Historically, natural products such as codeine (O-methylmorphine) have also been demethylated in poor to moderate yields by heating the ether in the presence of pyridinium chloride at 220 °C, a process that is sometimes called the Prey ether cleavage.[8]
Boron tribromide, which can be used at room temperature or below, is a more specialized reagent for the demethylation of aryl methyl ethers. The mechanism of ether dealkylation proceeds via the initial reversible formation of a Lewis acid-base adduct between the strongly Lewis acidic BBr3 and the Lewis basic ether (eq. 1). This Lewis adduct can reversibly dissociate to give a dibromoboryl oxonium cation and Br– (eq. 2). Rupture of the ether linkage occurs through the subsequent nucleophilic attack on the oxonium species by Br– to yield an aryloxydibromoborane and methyl bromide (eq. 3). Upon completion of the reaction, the phenol is liberated upon hydrolysis of the dibromoborane derivative during aqueous workup.[9]
- (Ar)(Me)O: + BBr3 ⇌ [(Ar)(Me)O+(BBr3–)]
- [(Ar)(Me)O+(BBr3–)] ⇌ (Ar)(Me)O+(BBr2) + :Br–
- (Ar)(Me)O+(BBr2) + :Br– → ArOBBr2 + MeBr
- ArOBBr2 + 3 H2O → ArOH + B(OH)3 + 2 HBr
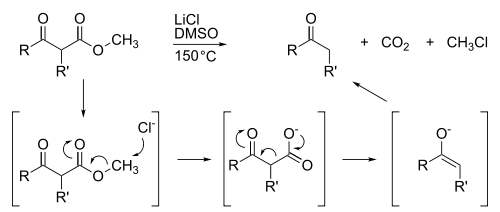
Methyl esters also are susceptible to demethylation, which is usually achieved by saponification. Highly specialized demethylations are abundant such as the Krapcho decarboxylation:
N-Demethylation
N-demethylation of 3° amines is by the von Braun reaction, which uses BrCN as the reagent to give the corresponding nor- derivatives. A modern variation of the Von Braun reaction was developed where BrCN was superseded by ethyl chloroformate. The preparation of Paxil from arecoline is an application of this reaction, as well as the synthesis of GSK-372,475, for example.
References
- ↑ Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. (2001). Organic Chemistry. Oxford, Oxfordshire: Oxford University Press. ISBN 0-19-850346-6.
- ↑ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 0-471-72091-7
- ↑ Roland Sigel; Sigel, Astrid; Sigel, Helmut (2007). The Ubiquitous Roles of Cytochrome P450 Proteins: Metal Ions in Life Sciences. New York: Wiley. ISBN 0-470-01672-8.
- ↑ W. Boerjan; J. Ralph; M. Baucher (June 2003). "Lignin biosynthesis". Annu. Rev. Plant Biol. 54 (1): 519–549. doi:10.1146/annurev.arplant.54.031902.134938. PMID 14503002.
- ↑ Irwin A. Pearl (1949). "Protocatechulic Acid". Organic Syntheses. 29: 85. ; Collective Volume, 3, p. 745
- ↑ Robert E. Ireland; David M. Walba (1977). "Demethylation of Methyl Aryl Ethers: 4-Ethoxy-3-hydroxybenzaldehyde". 56. doi:10.1002/0471264180.os056.11.
- ↑ Streitwieser, Andrew; Heathcock, Clayton H.; Kosower, Edward M. (1992). Introduction to organic chemistry (4th ed.). Upper Saddle River, N.J.: Prentice Hall. ISBN 0139738509. OCLC 52836313.
- ↑ Lawson, J. A.; DeGraw, J. I. (1977). "An improved method for O-demethylation of codeine". Journal of Medicinal Chemistry. 20 (1): 165–166. doi:10.1021/jm00211a037. ISSN 0022-2623.
- ↑ J. F. W. McOmie, D. E. West (1969). "3,3'-Dihydroxybiphenyl". Organic Syntheses. 49: 13. ; Collective Volume, 5, p. 412
See also
- Methylation, the addition of a methyl group to a substrate