PTPRE
Receptor-type tyrosine-protein phosphatase epsilon is an enzyme that in humans is encoded by the PTPRE gene.[5][6]
Function
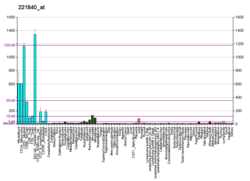
The protein encoded by this gene is a member of the protein tyrosine phosphatase (PTP) family. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. Two alternatively spliced transcript variants of this gene have been reported, one of which encodes a receptor-type PTP that possesses a short extracellular domain, a single transmembrane region, and two tandem intracytoplasmic catalytic domains; Another one encodes a PTP that contains a distinct hydrophilic N-terminus, and thus represents a nonreceptor-type isoform of this PTP. Studies of the similar gene in mice suggested the regulatory roles of this PTP in RAS related signal transduction pathways, cytokines induced SATA signaling, as well as the activation of voltage-gated K+ channels.[6]
Interactions
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000132334 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000041836 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ van den Maagdenberg AM, van den Hurk HH, Weghuis D, Wieringa B, Geurts van Kessel A, Hendriks WJ (April 1996). "Assignment of the human protein tyrosine phosphatase epsilon (PTPRE) gene to chromosome 10q26 by fluorescence in situ hybridization". Genomics. 30 (1): 128–9. doi:10.1006/geno.1995.0026. PMID 8595895.
- 1 2 "Entrez Gene: PTPRE protein tyrosine phosphatase, receptor type, E".
- ↑ Peretz A, Gil-Henn H, Sobko A, Shinder V, Attali B, Elson A (August 2000). "Hypomyelination and increased activity of voltage-gated K(+) channels in mice lacking protein tyrosine phosphatase epsilon". EMBO J. 19 (15): 4036–45. doi:10.1093/emboj/19.15.4036. PMC 306594. PMID 10921884.
Further reading
- Roskoski R (2005). "Src kinase regulation by phosphorylation and dephosphorylation". Biochem. Biophys. Res. Commun. 331 (1): 1–14. doi:10.1016/j.bbrc.2005.03.012. PMID 15845350.
- Krueger NX, Streuli M, Saito H (1990). "Structural diversity and evolution of human receptor-like protein tyrosine phosphatases". EMBO J. 9 (10): 3241–52. PMC 552056. PMID 2170109.
- Adams MD, Kerlavage AR, Fleischmann RD, Fuldner RA, Bult CJ, Lee NH, Kirkness EF, Weinstock KG, Gocayne JD, White O (1995). "Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence" (PDF). Nature. 377 (6547 Suppl): 3–174. PMID 7566098.
- Murakawa K, Matsubara K, Fukushima A, Yoshii J, Okubo K (1995). "Chromosomal assignments of 3'-directed partial cDNA sequences representing novel genes expressed in granulocytoid cells". Genomics. 23 (2): 379–89. doi:10.1006/geno.1994.1514. PMID 7835887.
- Okubo K, Itoh K, Fukushima A, Yoshii J, Matsubara K (1996). "Monitoring cell physiology by expression profiles and discovering cell type-specific genes by compiled expression profiles". Genomics. 30 (2): 178–86. doi:10.1006/geno.1995.9887. PMID 8586417.
- Elson A, Leder P (1996). "Identification of a cytoplasmic, phorbol ester-inducible isoform of protein tyrosine phosphatase epsilon". Proc. Natl. Acad. Sci. U.S.A. 92 (26): 12235–9. doi:10.1073/pnas.92.26.12235. PMC 40331. PMID 8618876.
- Gastier JM, Brody T, Pulido JC, Businga T, Sunden S, Hu X, Maitra S, Buetow KH, Murray JC, Sheffield VC, Boguski M, Duyk GM, Hudson TJ (1996). "Development of a screening set for new (CAG/CTG)n dynamic mutations". Genomics. 32 (1): 75–85. doi:10.1006/geno.1996.0078. PMID 8786123.
- Elson A, Kozak CA, Morton CC, Weremowicz S, Leder P (1997). "The protein tyrosine phosphatase epsilon gene maps to mouse chromosome 7 and human chromosome 10q26". Genomics. 31 (3): 373–5. doi:10.1006/geno.1996.0061. PMID 8838320.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Toledano-Katchalski H, Elson A (1999). "The transmembranal and cytoplasmic forms of protein tyrosine phosphatase epsilon physically associate with the adaptor molecule Grb2". Oncogene. 18 (36): 5024–31. doi:10.1038/sj.onc.1202883. PMID 10490839.
- Tanuma N, Nakamura K, Shima H, Kikuchi K (2000). "Protein-tyrosine phosphatase PTPepsilon C inhibits Jak-STAT signaling and differentiation induced by interleukin-6 and leukemia inhibitory factor in M1 leukemia cells". J. Biol. Chem. 275 (36): 28216–21. doi:10.1074/jbc.M003661200. PMID 10859312.
- Peretz A, Gil-Henn H, Sobko A, Shinder V, Attali B, Elson A (2000). "Hypomyelination and increased activity of voltage-gated K(+) channels in mice lacking protein tyrosine phosphatase epsilon". EMBO J. 19 (15): 4036–45. doi:10.1093/emboj/19.15.4036. PMC 306594. PMID 10921884.
- Gil-Henn H, Volohonsky G, Toledano-Katchalski H, Gandre S, Elson A (2000). "Generation of novel cytoplasmic forms of protein tyrosine phosphatase epsilon by proteolytic processing and translational control". Oncogene. 19 (38): 4375–84. doi:10.1038/sj.onc.1203790. PMID 10980613.
- Wabakken T, Hauge H, Finne EF, Wiedlocha A, Aasheim H (2002). "Expression of human protein tyrosine phosphatase epsilon in leucocytes: a potential ERK pathway-regulating phosphatase". Scand. J. Immunol. 56 (2): 195–203. doi:10.1046/j.1365-3083.2002.01126.x. PMID 12121439.
- Wabakken T, Hauge H, Funderud S, Aasheim HC (2002). "Characterization, expression and functional aspects of a novel protein tyrosine phosphatase epsilon isoform". Scand. J. Immunol. 56 (3): 276–85. doi:10.1046/j.1365-3083.2002.01127.x. PMID 12193229.
- Blanchetot C, Tertoolen LG, Overvoorde J, den Hertog J (2003). "Intra- and intermolecular interactions between intracellular domains of receptor protein-tyrosine phosphatases". J. Biol. Chem. 277 (49): 47263–9. doi:10.1074/jbc.M205810200. PMID 12376545.
- Tiran Z, Peretz A, Attali B, Elson A (2003). "Phosphorylation-dependent regulation of Kv2.1 Channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase epsilon". J. Biol. Chem. 278 (19): 17509–14. doi:10.1074/jbc.M212766200. PMID 12615930.
- Toledano-Katchalski H, Kraut J, Sines T, Granot-Attas S, Shohat G, Gil-Henn H, Yung Y, Elson A (2004). "Protein tyrosine phosphatase epsilon inhibits signaling by mitogen-activated protein kinases". Mol. Cancer Res. 1 (7): 541–50. PMID 12754301.