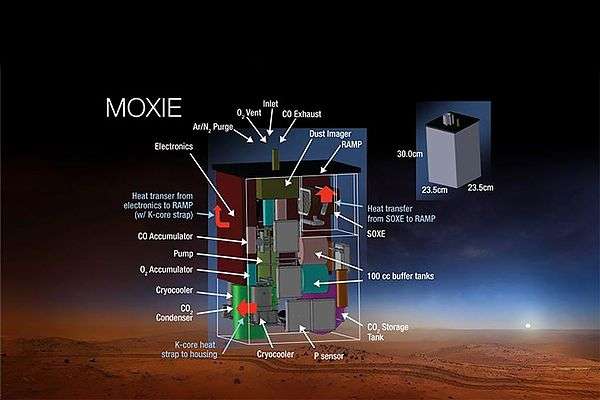
Mars Oxygen ISRU Experiment
| Operator | NASA |
|---|---|
| Manufacturer | MIT & Niels Bohr Institute |
| Instrument type | ISRU experimental technology |
| Function | oxygen production |
| Website |
mars |
| Properties | |
| Mass | 15 kg (33 lb) |
| Host Spacecraft | |
| Spacecraft | Mars 2020 rover |
| Launch date | July 2020 (planned) |
| Rocket | Atlas V 541 |
| Launch Site | Cape Canaveral SLC-41 |
MOXIE (Mars OXygen In situ resource utilization Experiment) is an exploration technology experiment that will produce a small amount of pure oxygen from Martian atmospheric carbon dioxide (CO2) in a process called solid oxide electrolysis.[1][2]
MOXIE is a 1% scale model aboard the planned Mars 2020 rover.[3] The Principal Investigator of the MOXIE instrument is Michael Hecht from the Massachusetts Institute of Technology (MIT).[1][4][5] The Niels Bohr Institute at the University of Copenhagen is collaborating with MIT to develop this prototype.[1][6] If successful, the technology can be scaled up as a means of producing oxygen for propellant oxidant in a Mars Ascent Vehicle (MAV) for a sample return.[7]
Objective
The main objective of this experiment is to produce molecular oxygen (O2) from the atmospheric carbon dioxide (CO2) present in the atmosphere at 96%.[1][8] Scientists will record the efficiency of the O2 production rate, and the resulting oxygen and carbon monoxide will be vented out after measurements are done.
To achieve this objective, the MOXIE instrument has a goal of producing 22 g of oxygen (O2) per hour with >99.6% purity during 50 sols (Martian days).[1][3][9]
NASA officials stated that if MOXIE worked efficiently, they could land a 100 times larger MOXIE-based instrument on Mars, along with a radioisotope thermoelectric generator. Over the course of some years the generator would power the system, which would produce up to two kilograms of oxygen per hour,[10] and fill an oxygen reservoir that could be used for a sample return mission,[11] or for when astronauts arrive sometime in the 2030s.[3][12] The stored oxygen could be used for life support, and can also be used as rocket propellant oxidizer to power their return trip to Earth.[13][14] High purity is crucial as future astronauts will breathe it.[15] N
2 and Ar are not separated from feed, but vented with carbon monoxide (CO). The CO, a byproduct of the reaction, may also be collected and used as propellant[16] or converted to methane (CH
4) for use as propellant.[2][17]
Development
| MOXIE | Units/performance[18][19] |
|---|---|
| Purpose | Test O 2 production from atmospheric CO 2 |
| Mass | 15 kg (33 lb) |
| Power | 300 W |
| Dimensions | 23.9 × 23.9 × 30.9 cm |
| O 2 production rate | 10-22 grams/hr |
The MOXIE experiment follows up on an earlier experiment, Mars ISPP Precursor ("MIP"), which was designed and built to fly on the Mars Surveyor 2001 Lander mission.[20] MIP was intended to demonstrate In-Situ Propellant Production ("ISPP") at a laboratory-scale using electrolysis of carbon dioxide to produce oxygen on Mars. The MIP experiment was postponed when the 2001 Lander mission was cancelled following the failure of the 1998 Mars Polar Lander.
The Principal Investigator is Michael Hecht from the Massachusetts Institute of Technology. Collaborators include the University of Copenhagen, Arizona State University, Imperial College of Science, NASA Jet Propulsion Laboratory, Ceramatec, Inc., Space Exploration Instruments LLC., and the Technical University of Denmark.[21]
Principle
MOXIE collects CO
2 from the Martian atmosphere, then electrochemically splits the CO
2 molecules into O
2 and CO. A solid oxide electrolysis cell works on the principle that, at elevated temperatures, certain ceramic oxides, such as yttria-stabilized zirconia (YSZ) and doped ceria, become oxide ion (O2–) conductors. A thin nonporous disk of YSZ (solid electrolyte) is sandwiched between two porous electrodes. For oxygen generation from carbon dioxide, CO2 diffuses through the porous electrode (cathode) and reaches the vicinity of the electrode-electrolyte boundary. Through a combination of thermal dissociation and electrocatalysis, an oxygen atom is liberated from the CO
2 molecule and picks up two electrons from the cathode to become an oxide ion (O2–). Via oxygen ion vacancies in the crystal lattice of the electrolyte, the oxygen ion is transported to the electrolyte-anode interface due to the applied DC potential. At this interface the oxygen ion transfers its charge to the anode, combines with another oxygen atom to form oxygen (O2), and diffuses out of the anode.
The net reaction is thus 2CO
2
2CO + O
2
See also
- Atmosphere of Mars
- Composition of Mars
- Exploration of Mars
- PIXL
- Project Morpheus (O2/methane engine)
- SHERLOC
References
- 1 2 3 4 5 "NASA TechPort -- Mars OXygen ISRU Experiment Project". NASA TechPort. National Aeronautics and Space Administration. Retrieved 19 November 2015.
- 1 2 Wall, Mike (August 1, 2014). "Oxygen-Generating Mars Rover to Bring Colonization Closer". Space.com. Retrieved 2014-11-05.
- 1 2 3 The Mars Oxygen ISRU Experiment (MOXIE) PDF. Presentation: MARS 2020 Mission and Instruments". November 6, 2014.
- ↑ Weinstock, Maia (July 31, 2014). "Going to the Red Planet". MIT News. Massachusetts Institute of Technology. Retrieved 2014-11-05.
- ↑ Weinstock, Maia (August 1, 2014). "Oxygen-creating instrument selected to fly on the upcoming Mars 2020 mission". Phys Org. Retrieved 2014-11-06.
- ↑ Brix, Lise (26 April 2015). "Scientists are trying to brew oxygen on Mars". Science Nordic. Retrieved 2015-05-15.
- ↑ Geoffrey A. Landis; Steven R. Oleson; Thomas W. Packard; Diane L. Linne; Jeffrey M. Woytach; Michael C. Martini; James E. Fittje; John Z. Gyekenyesi; Anthony J. Colozza; James Fincannon. (eds.). Design Study of a Mars Ascent Vehicle for sample return using in-situ generated propellant. 10th Symposium on Space Resource Utilization. doi:10.2514/6.2017-0424.
- ↑ Going to the Red Planet
- ↑ Dreier, Casey (31 July 2014). "NASA Selects 7 Science Instruments for its Next Mars Rover". The Planetary Society. Retrieved 2014-11-05.
- ↑ "Mars Colonization Edges Closer Thanks to MIT's Oxygen Factory". Sputnik International. 5 March 2015. Retrieved 2015-05-15.
- ↑ Design Study of a Mars Ascent Vehicle for Sample Return Using In-Situ Generated Propellant, Geoffrey A. Landis, Steven R. Oleson, Thomas W. Packard, Diane L. Linne, Jeffrey M. Woytach, Michael C. Martini, James E. Fittje, John Z. Gyekenyesi, Anthony J. Colozza, James Fincannon. 10th Symposium on Space Resource Utilization Grapevine, Texas. June 2017
- ↑ Maxey, Kyle (August 5, 2014). "Can Oxygen Be Produced on Mars? MOXIE Will Find Out". Engineering.com. Retrieved 2014-11-05.
- ↑ Thomson, Iain (31 July 2014). "Mars rover 2020: Oxygen generation and 6 more amazing experiments". The Register. Retrieved 2014-11-05.
- ↑ Living off the Land in the Final Frontier Archived 2014-11-06 at the Wayback Machine.. NASA, October 31, 2014.
- ↑ "MIT Developing Device to Produce Breathable Oxygen on Mars". The Space Reporter. 3 March 2015. Retrieved 2015-05-15.
- ↑ G. Landis and D. Linne, "A Mars Rocket Vehicle With In-situ Propellant Production", AIAA Journal of Spacecraft and Rockets, Vol. 38, No. 5, Sept.-Oct. 2001, pp. 730-735
- ↑ Ceramic Oxygen Generator for Carbon Dioxide Electrolysis Systems
- ↑ MOXIE - Overview. NASA. Accessed 11 August 2018.
- ↑ Oxygen Production from Mars Atmosphere Carbon Dioxide Using Solid Oxide Electrolysis. doi: 10.1149/07801.2953ecst, ECS Trans. 2017 volume 78, issue 1, 2953-2963
- ↑ D. Kaplan, R. Baird, H. Flynn, J. Rafliff, C. Baraona, P. Jenkins, G. Landis, D. Scheiman, K. Johnson, P. Karlmann, "The 2001 Mars In-Situ-Propellant-Production Precurson (MIP) Flight Demonstration: Project Objectives and Qualification Test Results", AIAA Space 2000 Conference and Exposition, paper AIAA-2000-5145, September 19–21, 2000, Long Beach CA. (abstract)
- ↑ MOXIE Team]. NASA. Accessed: 11 August 2018.