Chlamydia trachomatis
| Chlamydia trachomatis | |
|---|---|
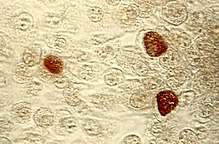 | |
| Chlamydia trachomatis in brown | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Chlamydiae |
| Class: | Chlamydiae |
| Order: | Chlamydiales |
| Family: | Chlamydiaceae |
| Genus: | Chlamydia |
| Species: | C. trachomatis |
| Binomial name | |
| Chlamydia trachomatis (Busacca 1935) Rake 1957 emend. Everett et al. 1999[1] | |
| Synonyms | |
| |
Chlamydia trachomatis (/kləˈmɪdiə
Different types of C. trachomatis cause different diseases. The most common strains cause disease in the genital tract, while other strains cause disease in the eye or lymph nodes. Like other Chlamydia species, the C. trachomatis life cycle consists of two morphologically distinct life stages: elementary bodies and reticulate bodies. Elementary bodies are spore-like and infectious. Reticulate bodies are the replicative stage and are only seen within host cells.
Description
Chlamydia trachomatis is a gram-negative bacteria that can only replicate within a host cell.[3] Over the course of the C. trachomatis life cycle, the bacteria take on two distinct forms. Elementary bodies are 200 to 400 nanometers across, and are surrounded by a rigid cell wall that allows them to survive outside of a host cell.[3][4] This form can initiate a new infection if it comes into contact with a susceptible host cell.[3] Reticulate bodies are 600 to 1500 nanometers across, and are only found within host cells.[4] Neither form is motile.[4]
The C. trachomatis genome is substantially smaller than that of many other bacteria at approximately 1.04 megabases, encoding approximately 900 genes.[3] A number of important metabolic functions are not encoded in the C. trachomatis genome, and instead are likely scavenged from the host cell.[3] In addition to the chromosome that contains most of the genome, nearly all C. trachomatis strains carry a 7.5 kilobase plasmid that contains 8 genes.[4] The role of this plasmid is unknown, though strains without the plasmid have been isolated, suggesting it is not required for suvival of the bacterium.[4]
Life cycle
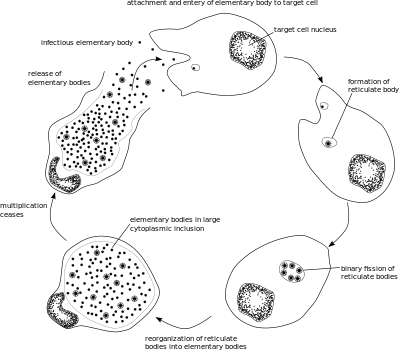
Like other Chlamydia species, C. trachomatis has a life cycle consisting of two morphologically distinct forms. First, C. trachomatis attaches to a new host cell as a small spore-like form called the elementary body.[5] The elementary body enters the host cell, surrounded by a host vacuole, called an inclusion.[5] Within the inclusion, C. trachomatis transforms into a larger, more metabolically active form called the reticulate body.[5] The reticulate body substantially modifies the inclusion, making it a more hospitable environment for rapid replication of the bacteria, which occurs over the following 30 to 72 hours.[5] The massive number of intracellular bacteria then transition back to resistant elementary bodies, before causing the cell to rupture and being released into the environment.[5] These new elementary bodies are then shed in the semen or released from epithelial cells of the female genital tract, and attach to new host cells.[6]
Classification
C. trachomatis are bacteria in the phylum Chlamydiae, a group of obligate intracellular parasites of eukaryotic cells.[3]
C. trachomatis strains are generally divided into three biovars based on the type of disease they cause. These are further subdivided into several serovars based on surface antigens recognized by the immune system.[3] Serovars A through C cause trachoma. Serovars D through K infect the genital tract, causing pelvic inflammatory disease, ectopic pregnancies, and infertility. Serovars L1 through L3 cause an invasive infection of the lymph nodes near the genitals, called lymphogranuloma venereum.[3]
C. trachomatis is thought to have diverged from other Chlamydia species around 6 million years ago, when its sole host modern humans emerged.[5] The closest relative to C. trachomatis is C. muridarum, which infects mice.[5] Different evolutionarily distinct lineages of C. trachomatis seem to cause disease in different body niches:[5]
|
Strains that cause lymphogranuloma venereum (Serovars L1 to L3) | |||||||||||||
|
| |||||||||||||
Role in disease
Clinical signs and symptoms of C. trachomatis infection and gonorrhea infection are indistinguishable.[7] Both are common causes of urethritis.[8] C. trachomatis is the single most important infectious agent associated with blindness (trachoma); about 84 million worldwide suffer C. trachomatis eye infections and 8 million are blinded as a result of the infection.[9]
Pathogenesis
Elementary bodies are generally present in the semen of infected men and vaginal secretions of infected women.[6] When they come into contact with a new host cell, the elementary bodies bind to the cell via interaction between adhesins on their surface and several host receptor proteins and heparan sulfate proteoglycans.[3] Once attached, the bacteria inject various effector proteins into the host cell using a type three secretion system.[3] These effectors trigger the host cell to take up the elementary bodies and prevent the cell from triggering apoptosis.[3] Within 6 to 8 hours after infection, the elementary bodies transition to reticulate bodies and a number of new effectors are synthesized.[3] These effectors include a number of proteins that modify the inclusion membrane, called Inc proteins, as well as proteins that redirect host vesicles to the inclusion.[3] 8 to 16 hours after infection, another set of effectors are synthesized, driving acquisition of nutrients from the host cell.[3] At this stage, the reticulate bodies begin to divide, causing the inclusion to expand.[3] If several elemenary bodies have infected a single cell, their inclusions will fuse at this point to create a single large inclusion in the host cell.[3] From 24 to 72 hours after infection, reticulate bodies transition to elementary bodies which are released either by lysis of the host cell or extrusion of the entire inclusion into the host genital tract.[3]
Presentation
Most people infected with C. trachomatis are asymptomatic. However, the bacteria can present in one of three ways: genitourinary (genitals), pulmonary (lungs), and ocular (eyes). Genitourinary cases can include genital discharge, vaginal bleeding, itchiness (pruritus), painful urination (dysuria), among other symptoms.[10] Often, symptoms are similar to those of a urinary tract infection.
Prevalence
Three times as many women as men are diagnosed with genitourinary C. trachomatis infections. Women aged 15–19 have the highest prevalence, followed by women aged 20–24, although the rate of increase of diagnosis is greater for men than for women. Risk factors for genitourinary infections include unprotected sex with multiple partners, lack of condom use, and living in an urban area.
Pulmonary infections can occur in infants born to women with active chlamydia infections, although the rate of infection is less than 10%.[10]
Ocular infections take the form of conjunctivitis or trachoma, both in adults and children. Trachoma is the primary source of infectious blindness in some parts of rural Africa and Asia[11] and is a neglected tropical disease that has been targeted by the World Health Organization for elimination by 2020.
Treatment
Treatment depends on the infection site, age of the patient, and whether another infection is present. Having a C. trachomatis and one or more other sexually transmitted infections at the same time is possible. Treatment is often done with both partners simultaneously to prevent reinfection. C. trachomatis may be treated with several antibiotic medications, including azithromycin, erythromycin, or ofloxacin.[7]
Azithromycin and doxycycline have equal efficacy to treat C. trachomatis with 97 and 98 percent success respectively. Treatment with doxycycline generic 2x1 100 mg for 7 days has equal success with expensive delayed-released doxycycline 1x 200 mg for 7 days.[12]
If treatment is necessary during pregnancy, levofloxacin, ofloxacin, and doxycycline are not prescribed. In the case of a patient who is pregnant, the medications typically prescribed are azithromycin, amoxicillin, and erythromycin. Amoxicillin has fewer side effects than the other medications for treating antenatal C. trachomatis infection. Retesting during pregnancy can be performed three weeks after treatment. If the risk of reinfection is high, screening can be repeated throughout pregnancy.[7]
If the infection has progressed, ascending the reproductive tract and pelvic inflammatory disease develops, damage to the fallopian tubes may have already occurred. In most cases, the C. trachomatis infection is then treated on an outpatient basis with azithromycin or doxycycline. Treating the mother of an infant with C. trachomatis of the eye, which can evolve into a pneumonia, is recommended.[7]
Laboratory tests
Chlamydia species are readily identified and distinguished from other Chlamydia species using DNA-based tests.
Most strains of C. trachomatis are recognized by monoclonal antibodies (mAbs) to epitopes in the VS4 region of MOMP.[13] However, these mAbs may also cross-react with two other Chlamydia species, C. suis and C. muridarum.
- Nucleic acid amplification tests (NAATs) tests find the genetic material (DNA) of Chlamydia bacteria. These tests are the most sensitive tests available, meaning they are very accurate and are very unlikely to have false-negative test results. A polymerase chain reaction (PCR) test is an example of a nucleic acid amplification test. This test can also be done on a urine sample.
- Nucleic acid hybridization tests (DNA probe test) also find Chlamydia DNA. A probe test is very accurate, but is not as sensitive as NAATs.
- Enzyme-linked immunosorbent assay (ELISA, EIA) finds substances (Chlamydia antigens) that trigger the immune system to fight Chlamydia infection. Chlamydia Elementary body (EB)-ELISA could be used to stratify different stages of infection based upon Immunoglobulin-γ status of the infected individuals [14]
- Direct fluorescent antibody test also finds Chlamydia antigens.
- Chlamydia cell culture is a test in which the suspected Chlamydia sample is grown in a vial of cells. The pathogen infects the cells, and after a set incubation time (48 hours), the vials are stained and viewed on a fluorescent light microscope. Cell culture is more expensive and takes longer (two days) than the other tests. The culture must be grown in a laboratory.[15]
Research
Due to its significance to human health, C. trachomatis is the subject of research in laboratories around the world. The bacteria are commonly grown in immortalised cell lines such as McCoy cells and HeLa cells.[4] Infectious particles can be quantified by infecting cell layers and counting the number of inclusions, analagous to a plaque assay.[4]
History
C. trachomatis was first described in 1907 by Stanislaus von Prowazek and Ludwig Halberstädter in scrapings from trachoma cases.[5] Thinking they had discovered a "mantled protozoan", they named the organism "Chlamydozoa" from the Greek "Chlamys" meaning mantle.[5] Over the next several decades, "Chlamydozoa" was thought to be a virus as it was small enough to pass through bacterial filters and unable to grow on known laboratory media.[5] However, in 1966 electron microscopy studies showed C. trachomatis to be a bacterium.[5]
C. trachomatis agent was first cultured in the yolk sacs of eggs by Tang Fei-fan, et al. in 1957.[16][17]
See also
References
- ↑ J.P. Euzéby. "Chlamydia". List of Prokaryotic names with Standing in Nomenclature. Archived from the original on 2008-03-23. Retrieved 2008-09-11.
- ↑ "Chlamydia trachomatis".
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Elwell C, Mirrashidi K, Engel J (2016). "Chlamydia cell biology and pathogenesis". Nature Reviews Microbiology. 14: 385–400. doi:10.1038/nrmicro.2016.30.
- 1 2 3 4 5 6 7 Kuo CC, Stephens RS, Bavoil PM, Kaltenboeck B (2015). "Chlamydia". In Whitman WB. Bergey's Manual of Systematics of Archaea and Bacteria. John Wiley & Sons. doi:10.1002/9781118960608.gbm00364. ISBN 9781118960608.
- 1 2 3 4 5 6 7 8 9 10 11 12 Nunes A, Gomes JP (2014). "Evolution, Phylogeny, and molecular epidemiology of Chlamydia". Infection, Genetics, and Evolution. 23: 49–64. doi:10.1016/j.meegid.2014.01.029.
- 1 2 Witkin SS, Minis E, Athanasiou A, Leizer J, Linhares IM (2017). "Chlamydia trachomatis:the persistent pathogen". Clinical and Vaccine Immunology. 24 (10): e00203–17. doi:10.1128/CVI.00203-17. PMID 28835360.
- 1 2 3 4 Malhotra M, Sood S, Mukherjee A, Muralidhar S, Bala M (September 2013). "Genital Chlamydia trachomatis: an update". Indian J. Med. Res. 138 (3): 303–16. PMC 3818592. PMID 24135174.
- ↑ Fredlund H, Falk L, Jurstrand M, Unemo M (2004). "Molecular genetic methods for diagnosis and characterisation of Chlamydia trachomatis and Neisseria gonorrhoeae: impact on epidemiological surveillance and interventions". APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 112 (11–12): 771–84. doi:10.1111/j.1600-0463.2004.apm11211-1205.x. PMID 15638837.
- ↑ "Trachoma". Prevention of Blindness and Visual Impairment. World Health Organization.
- 1 2 Mishori R, McClaskey EL, WinklerPrins VJ (15 December 2012). "Chlamydia Trachomatis Infections: Screening, Diagnosis, and Management". American Family Physician. 86 (12): 1127–1132. PMID 23316985.
- ↑ Global Network for Neglected Tropical Diseases. Trachoma interactive fact sheet.http://old.globalnetwork.org/sites/all/modules/globalnetwork/factsheetxml/disease.php?id=9. Accessed February 6, 2011
- ↑ "Chlamydial Infections in Adolescents and Adults". Retrieved January 23, 2017.
- ↑ Ortiz L, Angevine M, Kim SK, Watkins D, DeMars R (2000). "T-Cell Epitopes in Variable Segments of Chlamydia trachomatis Major Outer Membrane Protein Elicit Serovar-Specific Immune Responses in Infected Humans". Infect. Immun. 68 (3): 1719–23. doi:10.1128/IAI.68.3.1719-1723.2000. PMC 97337. PMID 10678996.
- ↑ Bakshi, Rakesh; Gupta, Kanupriya; Jordan, Stephen J.; Brown, LaDraka' T.; Press, Christen G.; Gorwitz, Rachel J.; Papp, John R.; Morrison, Sandra G.; Lee, Jeannette Y. (2017-04-21). "Immunoglobulin-Based Investigation of Spontaneous Resolution of Chlamydia trachomatis Infection". The Journal of Infectious Diseases. doi:10.1093/infdis/jix194. ISSN 1537-6613. PMID 28444306.
- ↑ "Chlamydia Tests". Sexual Conditions Health Center. WebMD. Retrieved 2012-08-07.
- ↑ Darougar S, Jones BR, Kinnison JR, Vaughan-Jackson JD, Dunlop EM (December 1972). "Chlamydial infection. Advances in the diagnostic isolation of Chlamydia, including TRIC agent, from the eye, genital tract, and rectum". Br J Vener Dis. 48 (6): 416–20. doi:10.1136/sti.48.6.416. PMC 1048360. PMID 4651177.
- ↑ Tang FF, Huang YT, Chang HL, Wong KC (1958). "Further studies on the isolation of the trachoma virus". Acta Virol. 2 (3): 164–70. PMID 13594716.
Tang FF, Chang HL, Huang YT, Wang KC (June 1957). "Studies on the etiology of trachoma with special reference to isolation of the virus in chick embryo". Chin Med J. 75 (6): 429–47. PMID 13461224.
Tang FF, Huang YT, Chang HL, Wong KC (1957). "Isolation of trachoma virus in chick embryo". J Hyg Epidemiol Microbiol Immunol. 1 (2): 109–20. PMID 13502539.
Further reading
Bellaminutti, Serena; Seracini, Silva; De Seta, Francesco; Gheit, Tarik; Tommasino, Massimo; Comar, Manola (November 2014). "HPV and Chlamydia trachomatis Co-Detection in Young Asymptomatic Women from High Incidence Area for Cervical Cancer". Journal of Medical Virology. 86 (11): 1920–1925. doi:10.1002/jmv.24041. Retrieved 13 November 2014.
External links
- Chlamydiae.com
- "Chlamydia trachomatis". NCBI Taxonomy Browser. 813.
- Type strain of Chlamydia trachomatis at BacDive - the Bacterial Diversity Metadatabase